![Abstract Image]() 6a-4 is TA 1887
6a-4 is TA 1887![]()
![Figure imgf000007_0001]()
TA 1887![]()
CAS 1003005-29-5![]()
Deleted CAS Registry Numbers: 1274890-87-7
C24 H26 F N O5
1H-Indole, 3-[(4-cyclopropylphenyl)methyl]-4-fluoro-1-β-D-glucopyranosyl-![]()
3-(4-cyclopropylbenzyl)-4-fluoroindole-N-glucoside![]()
(2R,3R,4S,5S,6R)-2-(3-(4-cvclopropylbenzyl)-4-fluoro-1 H-indol- 1 -yl)-6-(hvdroxymethyl)tetrahvdro-2H-pyran-3,4,5-triol,![]()
(TA-1887), a highly potent and selective hSGLT2 inhibitor, with pronounced antihyperglycemic effects in high-fat diet-fed KK (HF-KK) mice. Our results suggest the potential of indole-N-glucosides as novel antihyperglycemic agents through inhibition of renal SGLT2
Mitsubishi Tanabe Pharma Corp,![]()
Glucagon-like peptide-1 (GLP-I) is an incretin hormone that is released from L-cells in lower small intestine after food intake. GLP-I has been shown to stimulate glucose-dependent insulin secretion from pancreatic β-cells and increase pancreatic β-cell mass. GLP-I has also been shown to reduce the rate of gastric emptying and promote satiety. However, GLP-I is rapidly cleaved by dipeptidyl peptidase 4 (DPP4) leading to inactivation of its biological activity. Therefore, DPP4 inhibitors are considered to be useful as anti-diabetics or anti-obesity agents.
Sodium-glucose co-transporters (SGLTs) , primarily found in the intestine and the kidney, are a family of proteins involved in glucose absorption. Plasma glucose is filtered in the glomerulus and is reabsorbed by SGLTs in the proximal tubules. Therefore, inhibition of SGLTs cause excretion of blood glucose into urine and leads to reduction of plasma glucose level. In fact, it is confirmed that by continuous subcutaneous administration of an SGLT inhibitor, phlorizin, to diabetic animal models, the blood glucose level thereof can be normalized, and that by keeping the blood glucose level normal for a long time, the insulin secretion and insulin resistance can be improved [cf., Journal of Clinical Investigation, vol. 79, p. 1510 (1987); ibid., vol. 80, p. 1037 (1987); ibid., vol. 87, p. 561 (1991) ] .
In addition, by treating diabetic animal models with an SGLT inhibitor for a long time, insulin secretion response and insulin sensitivity of the animal models are improved without incurring any adverse affects on the kidney or imbalance in blood levels of electrolytes, and as a result, the onset and progress of diabetic nephropathy and diabetic neuropathy are prevented [cf., Journal of Medicinal Chemistry, vol. 42, p. 5311 (1999); British Journal of Pharmacology, vol. 132, p. 578 (2001)].
In view of the above, SGLT inhibitors are expected to improve insulin secretion and insulin resistance by decreasing the blood glucose level in diabetic patients and to prevent the onset and progress of diabetes mellitus and diabetic complications
DPP4 inhibitors are well known to those skilled in the art, and examples of DPP4 inhibitors can be found in the following publications: (1) TANABE SEIYAKU Co., Ltd.: WO 02/30891 or the corresponding U.S. patent (No. 6,849,622); and WO 02/30890 or the corresponding U.S. patent (No. 7,138,397); .
(2) Ferring BV: WO 95/15309, WO 01/40180, WO 01/81304, WO
01/81337, WO 03/000250, and WO 03/035057; (3) Probiodrug: WO 97/40832, EP1082314, WO 99/61431, WO
03/015775; (4) Novartis AG: WO 98/19998, WO 00/34241, WO 01/96295, US 6,107,317, US 6,110,949, and US 6,172,081;
(5) GlaxoSmithKline: WO 03/002531, WO 03/002530, and WO 03/002553; (6) Bristol Myers Squibb: WO 01/68603, WO 02/83128, and WO 2005/012249;
(7) Merck & Co.: WO 02/76450, and WO 03/004498;
(8) Srryx Inc.: WO 2005/026148, WO 2005/030751, WO 2005/095381, WO 2004/087053, and WO 2004/103993; (9) Mitsubishi Pharma Corp.: WO 02/14271, US 7,060,722, US
7,074,794, WO 2003/24942, Japan Patent Publication No.
2002-265439, Japan Patent Publication No. 2005-170792, and
WO 2006/088129;
(10) Taisho Pharma Co., Ltd.: WO 2004/020407; (12) Yamanouchi Pharmaceutical Co., Ltd.: WO 2004/009544,-
(13) Kyowa Hakko Kogyo : WO 02/051836;
(14) Kyorin Seiyaku: WO 2005/075421, WO 2005/077900, and WO 2005/082847;
(15) Alantos Pharmaceuticals: WO 2006/116157; (16) Glenmark Pharmaceuticals: WO 2006/090244, and WO 2005/075426;
(17) Sanwa Kagaku Kenkyusho : WO 2004/067509; and
(18) LG lifescience: WO 2005/037828, and WO 2006/104356.
In a preferable embodiment of the present invention, DPP4 inhibitors are the aliphatic nitrogen-containing 5- membered ring compounds disclosed in US 6,849,622, which are represented by Formula (29) :
…………………………………………..
WO 2012162115![]()
http://www.google.com/patents/EP2712359A2?cl=en![]()
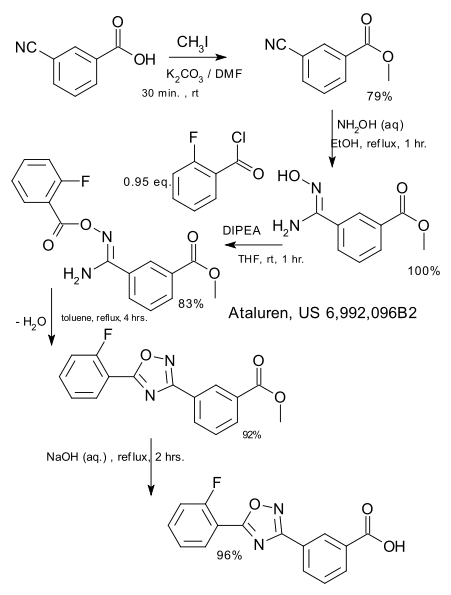
The present invention is further directed to a process for the preparation of a compound of formula (l-S)
(l-S)
(also known as 3-(4-cyclopropylbenzyl)-4-fluoro-1 -p-D-glucopyranosyl- 1 /-/-indole); or a pharmaceutically acceptable salt or prodrug thereof;
comprising
reacting a compound of formula (V-S), wherein PG1 is an oxygen protecting group with an acylating reagent; wherein the acylating reagent is present in an amount in the range of from about 1 .5 to about 3.0 molar equivalents; in the presence of a carbonyl source; in a first organic solvent; at a temperature in the range of from about room temperature to about 40°C; to yield the corresponding compound of formula (Vl-S);
reacting the compound of formula (Vl-S) with a compound of formula (Vll-S), wherein A1 is MgBr or MgCI; in an anhydrous organic solvent; to yield the corresponding compound of formula (Vlll-S);
reacting the compound of formula (Vlll-S) with a reducing agent; in the presence of a Lewis acid; in a second organic solvent; to yield the
corresponding compound of formula (IX-S);
Scheme 2.
![Figure imgf000058_0001]()
Example 1 : f2R.3R.4S.5R.6R)-2-facetoxymethyl)-6-f4-fluoro-3-formyl-1 H- indol-1 -yl)tetrahvdro-2H-pyran-3,4,5-triyl triacetate
![Figure imgf000077_0001]()
A 5-L 4-neck round bottom flask equipped with a thermocouple controller, mechanical stirrer, addition funnel, condenser, heating mantle, and a nitrogen inlet adapter was (2R,3R,4S,5R,6R)-2-(acetoxymethyl)-6-(4-fluoro-1 H- indol-1 -yl)tetrahydro-2H-pyran-3,4,5-triyl triacetate (225.0 g, 0.459 mol), DCE (1 .5 L) and DMF (50.2 ml_, 0.643 mol). The resulting mixture was warmed to 25°C, then phosphoryl chloride (107.8 ml_, 1 .15 mol) was added slowly via an addition funnel over 75 min. The resulting mixture was stirred for 30 min after the addition was completed, then slowly warmed to 40°C over 30 min, and then agitated at 40°C for an additional 12 h. The resulting solution was slowly poured into a rapidly stirred warm (40°C) 3M aqueous NaOAc (3.0 L) solution over 45 min. After the addition was completed, CH2CI2 (4.0 L) was added and the phases were separated. The aqueous phase was back extracted with CH2CI2 (1 .0 L) and the organic phases were combined, washed with 0.05 M HCI (2.0 L) and deionized water (2.0 L), then dried over MgS04. After filtration, the solvents were concentrated to dryness in vacuo to yield a solid, which was flushed with ethanol (1 .0 L) and re-evaporated. The resulting solid was transferred into a vacuum oven and dried at 40°C for 20 h to yield the title compound as a slightly yellow-brown solid.
1 H NMR (DMSO-d6, 300 MHz) δ 10.1 (s, 1 H), 8.53 (s, 1 H), 7.66 (d, J = 7.3 Hz, 1 H), 7.38 (m, 1 H), 7.10(dd, J = 6.7, 6.9 Hz, 1 H), 6.38 (d, J = 7.5 Hz, 1 H), 5.68 (dd, J = 6.5, 6.6 Hz, 1 H), 5.56 (t, J = 7.1 Hz, 1 H), 5.32 (t, J = 7.2 Hz, 1 H) 4.41 – 4.28 (m, 1 H), 4.24 – 4.06 (m, 2 H), 2.05 (s, 3H), 2.0 (s, 3H), 1 .98 (s, 3H), 1 .64 (s, 3H) 1JC NMR (DMSO-c(6, 75.47 MHz) £183.8, 169.9, 169.5, 169.3, 168.4, 155.8, 139.2, 135.7, 124.8, 1 17.7, 1 13.1 , 108.3, 107,9, 81 .9, 73.5, 72.1 , 70.3, 67.6, 61 .9, 20.4, 20.3, 20.1 , 19.6
LC-MS mlz MH+ = 494 (MH+), 516 [M+Na]+, 1009 [2M+Na]+
[a]D 25 = -0.099 (c = 0.316, CHCI3).
Example 2: f2R.3R.4S.5R.6R)-2-facetoxymethyl)-6-f3-ff4-cvclopropyl- phenyl)(hvdroxy)methyl)-4-fluoro-1 H-indol-1 -yl)tetrahydro-2H-pyran-3,4,5- triyl triacetate
![Figure imgf000078_0001]()
A 12-L 4-neck round bottom flask equipped with a mechanical stirrer, a thermocouple, a septum and nitrogen inlet adapter was charged with the compound prepared as in Example 1 (230 g, 0.457 mol) and anhydrous THF (4.2 L), and the resulting solution was cooled to 0°C with stirring under N2. A solution of freshly prepared (4-cyclopropylphenyl)magnesium bromide in THF (530 mL) was added dropwise via a double-tipped needle under gentle positive nitrogen pressure over 20 min, while the internal temperature was maintained between 0-8°C by adjusting the rate of addition. The resulting mixture was stirred at 0°C for 30 min. The reaction was quenched with saturated aqueous NH4CI solution (5.4 L) and then extracted with EtOAc (4 L, 3 L). The combined organic phase was washed with brine (2.7 L) and dried over MgS04. After filtration, the filtrate was concentrated at 66°C under house vacuum (-120 mmHg) followed by hi-vacuum (-20 mmHg) to yield a residue which contained a large amount of EtOAc, which residue was chased with ΟΗ2ΟΙ2 (800 mL) to yield the title compound as a yellowish solid, which was used in next step without further purification.
1 H NMR (DMSO-cfe, 300 MHz) δ 7.53 (dd, J = 7.9, 1 .1 Hz, 1 H), 7.41 (dd, J = 8.0, 1 .0 Hz, 1 H), 7.10-6.92 (m, 3 H), 6.78 (m, 1 H), 6.15 (m, 1 H), 5.92 (dd, J = 5.0, 4.1 Hz, 1 H), 5.65 (dd, J = 5.1 , 4.2 Hz, 1 H), 5.50 (m, 1 H), 5.24 (dd, J = 7.9, 8.3 Hz, 1 H), 4.38 – 4.22 (m, 1 H), 4.20-4.0 (m, 2 H), 2.05 (s, 3 H), 2.01 (s, 3 H), 1 .98 (s, 3 H), 1 .84 (m, 1 H), 0.92 (m, 2 H), 0.61 (m, 2 H)
13C NMR (DMSO-c/6, 75.47 MHz): £ 170.1 , 170.0, 169.9, 169.3, 156.1 , 140.9 139.0, 137.9, 128.0 (2 C), 125.2 (2 C), 124.2, 122.6, 1 16.3, 1 14.6, 107.4, 105.2, 81 .5, 76.8, 73.0, 72.6, 70.1 , 68.2, 62.0, 20.6, 20.4, 20.2, 19.8, 14.8, 8.96 (2 C)
LC-MS mlz MH+ = 612 (MH+), 634 [M+Na]+.
Example 3: (2R.3R.4S.5R.6R)-2-(acetoxymethyl)-6-(3-(4- cvclopropylbenzyl)-4-fluoro-1H-indol-1 -yl)tetrahvdro-2H-pyran-3,4.5-triyl triacetate
OAc
A 3-L 4-neck round bottom flask equipped with a mechanical stirrer, a thermocouple, a septum and nitrogen inlet adapter, was charged with the product prepared as in Example 2 above (82%, 334.6 g, 0.449 mol), DCE (1 .14 L), CH3CN (2.28 L), and Et3SiH (108.6 mL, 0.671 mol) and the resulting mixture was stirred and cooled to 0°C under N2. Boron trifluoride etherate (68.8 mL; 0.539 mol) was added dropwise over 10 min and the resulting mixture was stirred at 0°C for 30 minutes. After completion, saturated aqueous NaHCC>3 solution (4.2 L) was added to the mixture, which was extracted with EtOAc (5 L, 4 L) and the combined organic phase was dried over MgS04. After filtration, the filtrate was concentrated under house vacuum at 60°C to yield the title compound as a slightly yellowish solid.
The slightly yellowish solid (315.0 g) was triturated with EtOH (2.1 L, 200 proof) in a 4-L heavy duty Erlenmeyer flask at 76°C (with sonication x 3), and then gradually cooled to 20°C and stirred under N2 for 1 h. The solid was then collected by filtration and washed with cold (0°C) EtOH (200 ml_), dried by air- suction for 30 min, and then placed in a vacuum oven under house vacuum with gentle of N2 stream at 60°C for 18 h, to yield the title compound as an off- white crystalline solid.
1 H NMR (DMSO-de, 300 MHz) δ 7.47 (d, J = 8.3 Hz, 1 H), 7.22 (s, 1 H),
7.20-7.10 (m, 1 H), 7.06 (d, J = 8.1 , 2 H), 6.95 (d, J = 8.1 Hz, 1 H), 6.78 (dd, J = 7.1 , 7.0 Hz, 1 H), 6.16 (d, J = 7.1 Hz, 1 H), 5.61 -5.44 (m, 2 H), 5.21 (t, J = 7.3, 7.1 Hz, 1 H), 4.34 – 4.21 (m, 1 H), 4.18-4.04 (m, 2 H), 4.0 (s, 2 H), 2.04 (s, 3 H), 1 .97 (s, 3 H), 1 .95 (s, 3 H), 1 .84 (m, 1 H), 1 .63 (s, 3 H), 0.89 (m, 2 H), 0.61 (m, 2 H)
13C NMR (DMSO-d6, 75.47 MHz): £ 169.9, 169.5, 169.3, 168.3, 156.2, 140.9, 139.0, 137.9, 128.0 (2 C), 125.2 (2 C), 124.2, 122.7, 1 16.1 , 1 14.1 , 107.2, 105.0, 81 .7, 73.0, 72.5, 69.8, 68.0, 62.0, 31 .2, 20.4, 20.3, 20.2, 19.7, 14.6, 8.93 (2 C)
LC-MS mlz MH+ = 596 (MH+), 618 [M+Na]+, 1213 [2M+Na]+
[a]D 25 = -0.008 (c = 0.306, CHCI3).
Example 4: (2R.3R.4S.5S.6R)-2-(3-(4-cvclopropylbenzyl)-4-fluoro-1 H-indol- 1 -yl)-6-(hvdroxymethyl)tetrahvdro-2H-pyran-3,4,5-triol, ethanolate
OH
A 12-L 4-neck round bottom flask equipped with a mechanical stirrer, a thermocouple, a septum and nitrogen inlet adapter, was charged with the compound prepared as in Example 3 above (250 g, 0.413 mol), MeOH (1 .2 L) and THF (2.4 L), and the resulting mixture was stirred at 20°C under N2.
Sodium methoxide (2.5 ml_, 0.012 mol) solution was added dropwise and the resulting mixture was stirred at 20°C for 3 h. The solvent was concentrated at 60°C under house vacuum to yield a residue, which was dissolved in EtOAc (8.0 L), washed with brine (800 mL x 2) (Note 2), and dried over MgS04. The insoluble materials were removed by filtration, and the filtrate was concentrated at 60-66°C under hi-vacuum (20 mmHg) to yield the title compound as a slightly yellowish foamy solid.
The above obtained slightly yellowish foamy solid (195.1 g) was dissolved in EtOH (900 mL) at 76°C, and deionized H20 (1800 mL) was added slowly in a small stream that resulted in a slightly yellowish clear solution, which was then gradually cooled to 40°C with stirring while seeded (wherein the seeds were prepared, for example, as described in Example 5, below). The resulting slightly white-yellowish suspension was stirred at 20°C for 20 h, the solids were collected by filtration, washed with cold (0°C) EtOH/H20 (1 :4), and dried by air-suction for 6 h with gentle stream of N2 to yield the title compound as an off-white crystalline solid, as its corresponding EtOH/H20 solvate.
The structure of the EtOH/H20 solvate was confirmed by its 1H-NMR and LC-MS analyses. 1H-NMR indicated strong H20 and EtOH solvent residues, and the EtOH residue could not be removed by drying process. In addition, p-XRD of this crystalline solid showed a different pattern than that measured for a hemi-hydrate standard.
Example 5: (2R,3R,4S,5S,6R)-2-(3-(4-cvclopropylbenzyl)-4-fluoro-1 H-indol- 1 -yl)-6-(hvdroxymethyl)tetrahvdro-2H-pyran-3,4,5-triol, ethanolate
A 500-mL 3-neck round bottom flask equipped with a mechanical stirrer was charged with the compound prepared as in Example 3 above (4.67 g, 0.00784 mol), MeOH (47 mL) and THF (93 mL), and the resulting mixture was stirred at room temperature under argon atmosphere. Sodium methoxide (catalytic amount) solution was added dropwise and the resulting mixture was stirred at room temperature for 1 h. The solvent was concentrated at 30°C under reduced pressure. The residue was purified by silica gel column chromatography (chloroform : methanol = 99 : 1 – 90 : 10) to yield a colorless foamy solid (3.17 g).
First Crystallization
A portion of the colorless foamy solid prepared as described above (0.056 g) was crystallized from EtOH/H20 (1 :9, 5mL), at room temperature, to yield the title compound, as its corresponding EtOH solvate, as colorless crystals (0.047 g).
Second Crystallization
A second portion of the colorless foamy solid prepared as described above (1 .21 g) was dissolved in EtOH (6 mL) at room temperature. H20 (6 mL) was added, followed by addition of seeds (the colorless crystals, prepared as described in the first crystallization step above). The resulting suspension was stirred at room temperature for 18 h, the solids were collected by filtration, washed with EtOH/H20 (1 :4), and dried under reduced pressure to yield the title compound t, as its corresponding EtOH solvate, as an colorless crystalline solid (0.856 g).
The structure for the isolated compound was confirmed by 1H NMR, with peaks corresponding to the compound of formula (l-S) plus ethanol. Example 6: f2R.3R.4S.5S.6R)-2-f3-f4-cvclopropylbenzyl)-4-fluoro-1H-indol- 1 -yl)-6-(hvdroxymethyl)tetrahvdro-2H-pyran-3,4,5-triol hemihydrate
OH
A 5-L 4-neck round bottom flask equipped with a mechanical stirrer, a thermocouple, a septum and nitrogen inlet adapter was charged with the ethanolate (solvate) compound prepared as in Example 4 above (198.5 g, 0.399 mol) and deionized H20 (3.2 L). After the off-white suspension was warmed to 76°C in a hot water bath, along with sonication (x 4), it was gradually cooled to 20°C. The white suspension was stirred for 20 h at 20°C and then at 10°C for 1 h. The solid was collected by filtration, washed with deionized H20 (100 mL x 2), dried by air-suction for 2 h, and then placed in an oven under house vacuum with gentle stream of N2 at 50°C for 20 h, then at 60°C for 3 h to yield the title compound as an off-white crystalline solid.1 H NMR showed no EtOH residue and the p-XRD confirmed that the isolated material was a crystalline solid. TGA and DSC indicated that the isolated material contained about 2.3% of water (H20). M.P. = 108-1 1 1 °C.
1 H NMR (DMSO-c(6, 300 MHz) δ 7.36 (d, J = 8.2 Hz, 1 H), 7.22 (s, 1 H), 7.14 (d, J = 8.1 , 2 H), 7.10-7.0 (m, 1 H), 6.96 (d, J = 8.1 Hz, 2 H), 6.73 (dd, J = 7.5, 7.7 Hz, 1 H), 5.38 (d, J = 7.7 Hz, 1 H), 5.21 (d, J = 6.9 Hz, 1 H), 5.18 (d, J = 6.8 Hz, 1 H), 5.10 (d, J = 6.9 Hz, 1 H), 4.54 (t, J = 6.9, 1 .8 Hz, 1 H), 4.04 (s, 2 H), 3.75-3.60 (m, 2 H), 3.52-3.30 (m, 3 H), 3.20-3.17 (m, 1 H), 1 .84 (m, 1 H), 0.89 (m, 2 H), 0.61 (m, 2 H)
13C NMR (DMSO-de, 75.47 MHz): £ 156.2, 140.8, 139.4, 138.2, 128.2 (2 C), 125.2 (2 C), 124.4, 121 .8, 1 15.9, 1 12.8, 107.4, 104.2, 84.8, 79.3, 77.4, 71 .7, 69.8, 60.8, 31 .3, 14.6, 8.92 (2 C) LC-MS mlz MH+ = 428 (MH+), 450 [M+Na]+, 877 [2M+Na]+
[a]D 25 = -0.026 (c = 0.302, CH3OH)
Elemental Analysis: C2 H26NF05 + 0.54 H20 (MW = 437.20):
Theory: %C, 65.93; %H, 6.24; %N, 3.20; %F, 4.35, %H20, %2.23. Found: %C, 65.66; %H, 6.16; %N, 3.05; %F, 4.18, %H20, %2.26.
…………………………..
SEE
JP 2009196984![]()
……………………………………………..
WO 2008013322![]()
http://www.google.com/patents/WO2008013322A1?cl=en
Scheme 1 :
( III ) (ID
Scheme 2 :
( In the above scheme , R4 is bromine , or iodine , and the other symbols are the same as defined above.
The starting compounds of formula (V) can be prepared in accordance with the following scheme:
(V) (In the above scheme, the symbols are the same as defined above. )
The compounds of formula (XII ) can be prepared in accordance with the following scheme :
(In the above scheme, R5 is alkyl, and the other symbols are the same as defined above.)
Example 1 :
3- (4-Cyclopropylphenylmethyl) -4-fluoro-1- (β-D-gluco- pyranosyl) indole
OH
(1) A mixture of 4-fluoroindoline (185 mg) and D-glucose (267 mg) in H2O (0.74 ml) – ethyl alcohol (9 ml) was refluxed under argon atmosphere for 24 hours. The solvent was evaporated under reduced pressure to give crude 4-fluoro-1- (β-D-glucopyranosyl) indoline, whichwas used in the subsequent step without furtherpurification.
(2) The above compound was suspended in chloroform (8 ml) , and thereto were added successively pyridine (0.873 ml), acetic anhydride (1.02 ml) and 4- (dimethylamino) pyridine (a catalytic amount) . After being stirred at room temperature for 21 hours, the reaction solvent was evaporated under reduced pressure. The residue was dissolved in ethyl acetate , and the solution was washed witha 10 % aqueous copper (II) sulfate solutiontwice anda saturated aqueous sodium hydrogen carbonate solution, and dried over magnesium sulfate. The insoluble materials were filtered off, and the filtrate was evaporated under reduced pressure. The residue was purified by silica gel column chromatography (hexane : ethyl acetate = 90 : 10 – 60 : 40) to give 4-fluoro-1- (2, 3, 4, 6- tetra-O-acetyl-β-D-glucopyranosyl) indoline (365 mg) as colorless amorphous. APCI-Mass m/Z 468 (M+H) . 1H-NMR (DMSO-d6) δ 1.93 (s, 3H) , 1.96 (S1 3H) , 1.97 (s, 3H) , 2.00 (s, 3H) , 2.83 (ddd, J = 15.5, 10.5 and 10.3 Hz, IH) , 2.99 – 3.05 (m, IH) , 3.49 – 3.57 (m, 2H), 3.95 – 3.99 (m, IH), 4.07 – 4.11 (m, 2H), 4.95 (t, J = 9.5 Hz, IH) , 5.15 (t, J = 9.4 Hz, IH) , 5.42 (t, J= 9.6Hz, IH) , 5.49 (d, J= 9.3 Hz, IH) , 6.48 (t, J = 8.6 Hz, IH) , 6.60 (d, J = 8.0 Hz, IH) , 7.05 – 7.10 (m, IH) .
(3) The above compound (348 mg) was dissolved in 1,4-dioxane (14 ml), and thereto was added 2, 3-dichloro-5, 6-dicyano-l, 4- benzoquinone (306 mg) . After being stirred at room temperature for 33 hours , thereto was added a saturated aqueous sodium hydrogen carbonate solution (20 ml) , and the organic solvent was evaporated under reduced pressure. The residue was extracted with ethyl acetate twice, and the combinedorganic layerwas washedwithbrine, dried over magnesium sulfate and treated with activated carbon. The insoluble materials were filtered off, and the filtrate was evaporated under reduced pressure. The residue was purified by silica gel column chromatography (hexane : ethyl acetate = 90 : 10 – 60 : 40) and recrystallization from ethyl alcohol to give 4-fluoro-1- (2,3,4, 6-tetra-O-acetyl-β-D-glucopyranosyl) indole (313 mg) as colorless crystals, mp 132-135°C. APCI-Mass m/Z 483 (M+NH4) . 1H-NMR (DMSO-d6) δ 1.64 (s, 3H), 1.97 (s, 3H), 1.99 (s, 3H), 2.04 (S, 3H), 4.10 (ABX, J = 12.4, 2.7 Hz, IH), 4.14 (ABX, J = 12.4, 5.2 Hz, IH) , 4.31 (ddd, J = 10.0, 5.2 and 2.7 Hz, IH) , 5.25 (t, J = 9.7 Hz, IH) , 5.53 (t, J = 9.5 Hz, IH) , 5.61 (t, J = 9.3 Hz, IH) , 6.22 (d, J = 9.0 Hz, IH) , 6.58 (d, J = 3.4 Hz, IH) , 6.88 (dd, J = 10.8, 7.9 Hz, IH) , 7.19 (td, J = 8.1, 5.3 Hz, IH) , 7.51 (d, J“ = 8.5 Hz, IH) , 7.53 (d, J = 3.4 Hz, IH) . (4) The above compound (3.50 g) and N, N-dimethylformamide (3.49 ml) were dissolved in 1, 2-dichloroethane (70 ml) , and thereto was added dropwise phosphorus (III) oxychloride (2.10 ml) . The mixture was stirred at 7O0C for 1 hour, and thereto was added water (100 ml) at 00C. The resultant mixture was extracted with ethyl acetate (200 ml) twice, and the combined organic layer was washed with brine (40 ml) and dried over magnesium sulfate. The insoluble materials were filtered off, and the filtrate was evaporated under reduced pressure. The residue was purified by silica gel column chromatography (hexane : ethyl acetate = 90 : 10 – 50 : 50) and recrystallization from ethyl alcohol (20 ml) to give
4-fluoro-1- (2,3,4, 6-tetra-O-acetyl-β-D-glucopyranosyl) – indole-3 -carboxaldehyde (2.93 g) as colorless crystals, tnp 190 – 192°C. APCI-Mass m/Z 511 (M+NH4) . 1H-NMR (DMSO-de) δ 1.64 (s,
3H), 1.98 (s, 3H), 2.00 (s, 3H), 2.05 (s, 3H), 4.12 (A part of
ABX, J = 12.4, 2.5 Hz, IH) , 4.17 (B part of ABX, «7 = 12.4, 5.5
Hz, IH) , 4.33 (ddd, J= 10.0, 5.5 and 2.5 Hz, IH) , 5.32 (t, J= 9.8 Hz, IH) , 5.56 (t, J = 9.6 Hz, IH) , 5.66 (t, J = 9.3 Hz, IH) ,
6.36 (d, J = 9.0 Hz, IH) , 7.11 (dd, J = 10.6, 8.0 Hz, IH) , 7.38
(td, J = 8.1, 5.1 Hz, IH) , 7.65 (d, J = 8.3 Hz, IH) , 8.53 (s, IH) ,
10.0 (d, J = 2.9 Hz, IH) .
(5) To a mixture of magnesium turnings (664 mg) and 1, 2-dibromoethane (one drop) in tetrahydrofuran (40 ml) was added dropwise a solution of l-bromo-4-cyclopropylbenzene (see WO 96/07657) (5.2Ig) in tetrahydrofuran (12 ml) over 25 minutes under being stirred vigorously, and the mixture was vigorously stirred for 30 minutes at room temperature. The resultant mixture was then dropwise added to a solution of the above 4-fluoro-1- (2 , 3 , 4, 6- tetra-O-acetyl-β-D-glucopyranosyl) indole-3 -carboxaldehyde (4.35 g) in tetrahydrofuran (130 ml) over 15 minutes at -780C under argon atmosphere . The mixture was stirred at same temperature for 30 minutes, and thereto was added a saturated aqueous ammonium chloride solution (200 ml) . The resultant mixture was extracted with ethyl acetate (150 ml) twice, and the combined organic layer was dried over magnesium sulfate. The insoluble materials were filtered off, and the filtrate was evaporated under reduced pressure to give crude 4-cyclopropylphenyl 4-fluoro-l- (2,3,4, 6-tetra-O-acetyl-β-D-glucopyranosyl) indol-3-yl methanol, which was used in the subsequent step without further purification.
(6) To a stirred solution of the above compound and triethylsilane (2.11 ml) in dichloromethane (44 ml) – acetonitrile (87 ml) was added boron trifluoride -diethyl ether complex (1.34 ml) at O0C under argon atmosphere . The mixture was stirred at same temperature for 20 minutes, and thereto was added a saturated aqueous sodium
m/Z 479/481 (M+NH4) . 1H-NMR (DMSO-d6) δ 0.59 – 0.62 (m, 2H) , 0.88
- 0.91 (m, 2H) , 1.83 – 1.87 (m, IH) , 3.21 – 3.50 (m, 4H) , 3.57
- 3.63 (m, IH) , 3.65 – 3.71 (m, IH) , 4.18 (s, 2H) , 4.54 (t, J = 5.5 Hz, IH) , 5.10 (d, J = 5.3 Hz, IH) , 5.16 (d, J = 5.0 Hz, IH) , 5.23 (d, J“ = 5.8 Hz, IH) , 5.38 (d, J“ = 9.0 Hz, IH) , 6.97 (d, J“ = 8.2 Hz, 2H) , 7.01 (dd, J“ = 9.4, 2.0 Hz, IH) , 7.08 (d, J“ = 8.0 Hz, 2H) , 7.22 (s, IH) , 7.47 (dd, J = 10.1, 2.1 Hz, IH) .
……………………………………………………………..
US 20110065200![]()
http://www.google.com/patents/US20110065200![]()
Glucose analogs have long been used for the study of glucose transport and for the characterization of glucose transporters (for review, see Gatley (2003) J Nucl Med. 44(7):1082-6). Alpha-methylglucoside (AMG) is often the analog of choice for cell-based assays designed to study the activity of SGLT1 and/or SGLT2.
……………………………………..
WO 2009091082![]()
http://www.google.com/patents/WO2009091082A1?cl=en![]()
![Figure imgf000067_0001]() R1 = FLUORO, R2= H
R1 = FLUORO, R2= H![]()
……………….
Novel Indole-N-glucoside, TA-1887 As a Sodium Glucose Cotransporter 2 Inhibitor for Treatment of Type 2 Diabetes ![]()
(ACS Medicinal Chemistry Letters) Thursday November 21st 2013![]()
Author(s): Sumihiro Nomura, Yasuo Yamamoto, Yosuke Matsumura, Kiyomi Ohba, Shigeki Sakamaki, Hirotaka Kimata, Keiko Nakayama, Chiaki Kuriyama, Yasuaki Matsushita, Kiichiro Ueta, Minoru Tsuda-Tsukimoto,
DOI:10.1021/ml400339b
GO TO: [Article]![]()
http://pubs.acs.org/doi/full/10.1021/ml400339b![]()
………………
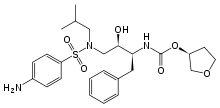
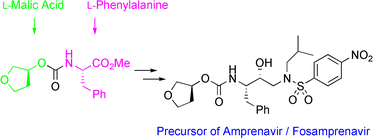
Organic Process Research & Development (2012), 16(11), 1727-1732.![]()
![Abstract Image]()
A practical synthesis of two N-glycoside indoles 1 and 2, identified as highly potent sodium-dependent glucose transporter (SGLT) inhibitors is described. Highlights of the synthetic process include a selective and quantitative Vilsmeier acylation and a high-yielding Grignard coupling reaction. The chemistry developed has been applied to prepare two separate SGLT inhibitors 1 and 2 for clinical evaluation without recourse to chromatography.
http://pubs.acs.org/doi/abs/10.1021/op3001355![]()
Preparation of (2R,3R,4S,5S,6R)-2-(3-(4-Cyclopropylbenzyl)-4-fluoro-1H-indol-1-yl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol (1)
To a solution of compound 6 (250 g, 0.413 mol) in MeOH (1.2 L) and THF (2.4 L) was added sodium methoxide (2.5 mL, 0.012 mol), ………….DELETED………………….. There was obtained 198.5 g (97.5% isolated yield based on free base form; 98.8 LCAP) of 1 EtOH/H2O solvate as an off-white crystalline solid. A slurry of the EtOH/H2O solvate 1 (198.5 g, 0.399 mol) in de-ionized H2O (3.2 L,) was warmed to 76 °C, and then the slurry was gradually cooled to 20 °C over 30 min. The white suspension was stirred at 20 °C for 20 min and then at 10 °C for 1 h. The solid was collected by filtration, washed with de-ionized H2O (100 mL × 2), dried in an oven at 50 °C for 20 h and further at 60 °C for 3 h to afford 177.4 g, (99.8% isolated yield, 98.6 LCAP) of 1 hemihydrate as an off-white crystalline solid, of which the 1H NMR showed no EtOH residue and the powder X-ray diffraction (pXRD) confirmed that it was a crystalline solid. TGA indicated it contained 2.3% of water.
Mp = 108–111 °C.
1H NMR (DMSO-d6, 300 MHz) δ 7.36 (d, J = 8.2 Hz, 1 H), 7.22 (s, 1 H), 7.14 (d, J = 8.1, 2 H), 7.10–7.0 (m, 1 H), 6.96 (d, J = 8.1 Hz, 2 H), 6.73 (dd, J = 7.5, 7.7 Hz, 1 H), 5.38 (d, J = 7.7 Hz, 1 H), 5.21 (d, J = 6.9 Hz, 1 H), 5.18 (d, J = 6.8 Hz, 1 H), 5.10 (d, J = 6.9 Hz, 1 H), 4.54 (t, J = 6.9, 1.8 Hz, 1 H), 4.04 (s, 2 H), 3.75–3.60 (m, 2 H), 3.52–3.30 (m, 3 H), 3.20–3.17 (m, 1 H), 1.84 (m, 1 H), 0.89 (m, 2 H), 0.61 (m, 2 H).
13C NMR (DMSO-d6, 75.47 MHz): δ 156.2, 140.8, 139.4, 138.2, 128.2 (2 C), 125.2 (2 C), 124.4, 121.8, 115.9, 112.8, 107.4, 104.2, 84.8, 79.3, 77.4, 71.7, 69.8, 60.8, 31.3, 14.6, 8.92 (2 C). LC–MS m/z MH+ = 428 (MH+), 450 [M + Na]+, 877 [2M + Na]+.
[α]25D = −0.026 (c = 0.302, CH3OH).
Anal. Calc’d for C24H26NFO5·0.54 H2O: C, 65.93; H, 6.24; N, 3.20; F, 4.35, H2O, 2.23. Found: C, 65.66; H, 6.16; N, 3.05; F, 4.18, H2O, 2.26.
…………………
Journal of Medicinal Chemistry (2010), 53(24), 8770-8774
http://pubs.acs.org/doi/abs/10.1021/jm101080v![]()
………………….
TETRAACETYL COMPD![]()
Organic Process Research & Development (2012), 16(11), 1727-1732.
http://pubs.acs.org/doi/full/10.1021/op3001355![]()
1003005-35-3
- C32 H34 F N O9
- 1H-Indole, 3-[(4-cyclopropylphenyl)methyl]-4-fluoro-1-(2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl)-
-
Preparation of (2R,3R,4S,5R,6R)-2-(acetoxymethyl)-6-(3-(4-cyclopropylbenzyl)-4-fluoro-1H-indol-1-yl)tetrahydro-2H-pyran-3,4,5-triyl Triacetate (6)
To a stirred solution of
5 (82%, 334.6 g, 0.449 mol) in DCE (1.14 L) and MeCN (2.28 L) at 0 °C was added Et
3SiH (108.6 mL, 0.671 mol) followed by the addition of boron trifluoride etherate (68.8 mL, 0.539 mol) ———DELETE………………….. There was obtained 228.6 g (85% isolated yield, 98.4 LCAP) of pure
6 as an off-white crystalline solid. Mp 168–169 °C.
1H NMR (DMSO-
d6, 300 MHz) δ 7.47 (d,
J = 7.2 Hz, 1H), 7.22 (s, 1H), 7.20–7.10 (m, 1H), 7.06 (d,
J = 8.1, 2H), 6.95 (d,
J = 8.1 Hz, 2H), 6.78 (dd,
J = 7.3, 7.0 Hz, 1H), 6.16 (d,
J = 7.1 Hz, 1H), 5.61–5.48 (m, 2H), 5.21 (t,
J = 7.3, 7.1 Hz, 1H), 4.34 – 4.25 (m, 1H), 4.18–4.04 (m, 2H), 4.0 (s, 2H), 2.04 (s, 3H), 1.97 (s, 3H), 1.95 (s, 3H), 1.84 (m, 1H), 1.61 (s, 3H), 0.89 (m, 2H), 0.61 (m, 2H).
13C NMR (DMSO-
d6, 75.47 MHz): δ 169.9, 169.5, 169.3, 168.3, 156.2, 140.9, 139.0, 137.9, 128.0 (2 C), 125.2 (2 C), 124.2, 122.7, 116.1, 114.1, 107.2, 105.0, 81.7, 73.0, 72.5, 69.8, 68.0, 62.0, 31.2, 20.4, 20.3, 20.2, 19.7, 14.6, 8.93 (2 C). HRMS:
m/
z = 596.2261 [M – 1]
+. [α]
25D = −0.008 (
c = 0.306, CHCl
3).
![]()
| WO2005012326A1 * |
Jul 30, 2004 |
Feb 10, 2005 |
Tanabe Seiyaku Co |
Novel compounds having inhibitory activity against sodium-dependant transporter |
| WO2006035796A1 * |
Sep 28, 2005 |
Apr 6, 2006 |
Norihiko Kikuchi |
1-(β-D-GLYCOPYRANOSYL)-3-SUBSTITUTED NITROGENOUS HETEROCYCLIC COMPOUND, MEDICINAL COMPOSITION CONTAINING THE SAME, AND MEDICINAL USE THEREOF |
| WO2010092124A1 * |
Feb 11, 2010 |
Aug 19, 2010 |
Boehringer Ingelheim International Gmbh |
Pharmaceutical composition comprising linagliptin and optionally a sglt2 inhibitor, and uses thereof |
| WO2010092125A1 * |
Feb 11, 2010 |
Aug 19, 2010 |
Boehringer Ingelheim International Gmbh |
Pharmaceutical composition comprising a sglt2 inhibitor, a dpp-iv inhibitor and optionally a further antidiabetic agent and uses thereof |
| WO2011143296A1 * |
May 11, 2011 |
Nov 17, 2011 |
Janssen Pharmaceutica Nv |
Pharmaceutical formulations comprising 1 – (beta-d-glucopyranosyl) – 2 -thienylmethylbenzene derivatives as inhibitors of sglt |
| US8163704 |
Oct 18, 2010 |
Apr 24, 2012 |
Novartis Ag |
Glycoside derivatives and uses thereof |
| US8466114 |
Mar 21, 2012 |
Jun 18, 2013 |
Novartis Ag |
Glycoside derivatives and uses thereof |
| WO2009091082A1 * |
Jan 16, 2009 |
Jul 23, 2009 |
Mitsubishi Tanabe Pharma Corp |
Combination therapy comprising sglt inhibitors and dpp4 inhibitors |
| WO2009117421A2 * |
Mar 17, 2009 |
Sep 24, 2009 |
Kalypsys, Inc. |
Heterocyclic modulators of gpr119 for treatment of disease |
| WO2011048148A2 |
Oct 20, 2010 |
Apr 28, 2011 |
Novartis Ag |
Glycoside derivative and uses thereof |
| WO2012089633A1 * |
Dec 22, 2011 |
Jul 5, 2012 |
Sanofi |
Novel pyrimidine derivatives, preparation thereof, and pharmaceutical use thereof as akt(pkb) phosphorylation inhibitors |
| WO2012162113A1 * |
May 18, 2012 |
Nov 29, 2012 |
Janssen Pharmaceutica Nv |
Process for the preparation of compounds useful as inhibittors of sglt-2 |
| WO2012162115A2 * |
May 18, 2012 |
Nov 29, 2012 |
Janssen Pharmaceutica Nv |
Process for the preparation of compounds useful as inhibitors of sglt-2 |
| WO2013090550A1 * |
Dec 13, 2012 |
Jun 20, 2013 |
National Health Research Institutes |
Novel glycoside compounds |
| US7666845 |
Dec 3, 2007 |
Feb 23, 2010 |
Janssen Pharmaceutica N.V. |
Compounds having inhibitory activity against sodium-dependent glucose transporter |
| US8394772 |
Oct 20, 2010 |
Mar 12, 2013 |
Novartis Ag |
Glycoside derivative and uses thereof |
| US8697658 |
Dec 13, 2012 |
Apr 15, 2014 |
National Health Research Institutes |
Glycoside compounds |
Filed under:
DIABETES,
Uncategorized Tagged:
DIABETES,
Mitsubishi Tanabe Pharma Corp,
SGLT inhibitors,
TA 1887 ![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()




















 6a-4 is TA 1887
6a-4 is TA 1887
























 R1 = FLUORO, R2= H
R1 = FLUORO, R2= H


























.gif)












 3p is compd
3p is compd











 22.9 kg) was added until a pH of 6.5 was attained. After agitating for 15 min and holding for 30 min, the aqueous layer was discarded, and the organic layer was washed with H2O (470 kg). The solution was then polish filtered, and isopropylacetate (52.2 kg) was used to rinse the polish filter assembly. The solution was concentrated under reduced pressure (240 Torr) to a volume of 718 L at <45 °C. Seeds (500 g) were charged, and the distillation was continued until a volume of
22.9 kg) was added until a pH of 6.5 was attained. After agitating for 15 min and holding for 30 min, the aqueous layer was discarded, and the organic layer was washed with H2O (470 kg). The solution was then polish filtered, and isopropylacetate (52.2 kg) was used to rinse the polish filter assembly. The solution was concentrated under reduced pressure (240 Torr) to a volume of 718 L at <45 °C. Seeds (500 g) were charged, and the distillation was continued until a volume of 






























































 trifarotene
trifarotene

