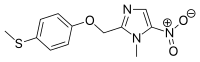
Fexinidazole, Hoe-239
1-Methyl-2-{[4-(methylsulfanyl)phenoxy]methyl}-5-nitro-1H-imidazole
| cas 59729-37-2 |
| Molecular formula | C12H13N3O3S |
| Molar mass | 279.31 g mol−1 |
Sanofi (Originator)
University of Dundee
Drugs for Neglected Diseases Initiative
Winkelmann, E.; Raether, W.
Chemotherapeutically active nitro compounds. 4,5-nitroimidazoles. Part III
Arzneim-Forsch Drug Res 1978, 28(5): 739
US 4042705, DE 2531303,
Fexinidazole is an antiparasitic agent.[1] It has activity against Trypanosoma cruzi, Tritrichomonas foetus, Trichomonas vaginalis,Entamoeba histolytica,[1] Trypanosoma brucei,[2] and Leishmania donovani.[3] The biologically relevant active metabolites in vivo are the sulfoxide and sulfone [3][4]
Fexinidazole was discovered by the German pharmaceutical company Hoechst AG, but its development as a pharmaceutical was halted in the 1980s.[5] Fexinidazole is now being studied through a collaboration between Sanofi and the Drugs for Neglected Diseases Initiative for the treatment of Chagas disease and human African trypanosomiasis (sleeping sickness).[6][7] Fexinidazole is the first drug candidate for the treatment of advanced-stage sleeping sickness in thirty years.[8]
Fexinidazole is currently in phase II/III clinical development at Drugs for Neglected Diseases Initiative for the oral treatment of African trypanosomiasis (sleeping sickness). In May 2009, Sanofi (formerly known as sanofi-aventis) licensed the drug candidate to Drugs for Neglected Diseases Initiative for the development, manufacturing and distribution as a treatment of human African trypanosomiasis. Once approved, the companies plan to make the drug available on a nonprofit basis.
Fexinidazole was originally developed by a German pharmaceutical company called Hoechst, now part of Sanofi; however, its development was abandoned in the 1980s when the company gave up its tropical disease programs. Fexinidazole is one of a class of drugs known as azoles, like fluconazole, that work against fungi and may work against cancer.
-
Onset of trypanosomiasis is caused by Trypanosoma protozoa and it is said that every year 200,000 to 300,000 of new patients of African sleeping sickness fall sick. At present the number of patients of African sleeping sickness cannot be confirmed due to the low reliability of the investigative data. According to the WHO, at least 150,000 people died of African sleeping sickness in 1996 and it is said that its aftereffect remains in not less than 100,000 people. Beyond that, enormous is the damage to domestic animals caused by a disease called as nagana, and several hundred thousands of cattle which are to be protein sources for people die every year. Further, in the area of about 10,000,000 km2of savanna equal to the United States of America, cattle-breeding is impossible due to Trypanosoma. Thus, African sleeping sickness remarkably damages the health and the economical development of African people, and this is the reason why the WHO adopts the trypanosomiasis as one of the infectious diseases that should be controlled.
-
African sleeping sickness is a protozoal infectious disease by Trypanosoma transmitted through tsetse flies and the protozoa appear in the blood stream in about 10 days after infection. In the initial period of infection the protozoa multiply in the blood stream and give fever, physical weakness, headache, a pain of muscles and joints and a feeling of itching to proceed. On entering the chromic period, the central nerve is affected to show symptoms such as mental confusion and systemic convulsion, and finally the patients lapse into lethargy and are led to death.
-
The trypanosomiasis of domestic animals has Trypanosoma brucei brucei, Trypanosoma evansi, Trypanosoma congolense and Trypanosoma vivax as pathogens and is a communicable disease which affects domestic animals such as horses, cattle, pigs and dogs and, in addition, mice, guinea pigs, rabbits and the like. Particularly, the loss of cattle and horses is greatest and almost fetal, and they are led to anemia, edema, weakening and the like and fall dead in one month after infection.
-
In treating trypanosomiasis, pentamidine, melarsoprol, eflornithine and the like are used and there was a feeling in the 1960s that its eradication might be possible. However, these drugs are old and are gradually losing their efficacy. Particularly, the resistance to melarsoprol of an arsenic agent causes a big problem and the situation is so dire that patients with no efficacy only await death and the development of novel antitrypanosoma agents are strongly desired.
-
Trypanosoma mainly lives in the blood stream of the human body. This bloodstream energy metabolism depends on the glycolytic pathway localized in the organelle characteristic of the protozoa which is called as glycosome and the so-called oxidative phosphorylation does not function. However, in order to efficiently drive this glycolytic pathway, the produced NADH has to be reoxidized, and the glycerol-3-phosphate oxidation system of mitochondria plays an important role in this reoxidation. The terminal oxidase of this oxidation system functions as a quinol oxidase having a reduced ubiquinone as an electron donor and has properties greatly different from those of cytochrome oxidase of an aerobic respiration system which the host has. Particularly, a remarkable point is that the terminal oxidase of the oxidation system is non-sensitive to the cyanide which quickly inhibits the cytochrome oxidase of the host. Then, many researchers centered around Western countries have tried to develop drugs targeting this cyanide resistant oxidase but effective drugs having a selective toxicity have not been obtained.
-
Under these circumstances the present inventors et al. found that isoprenoid based physiologically active substances of ascochlorin, ascofuranone and derivatives thereof, particularly ascofuranone specifically inhibits the glycerol-3-phosphate oxidation system of trypanosome at a very low concentration of the order of nM and filed a patent application (Japanese Patent Publication A No. : H09-165332). They also clarified that acofuranone exhibits a very strong multiplication inhibition effect in the copresence of glycerin (Molecular and Biochemical Parasitology, 81: 127-136, 1996).
In consideration of practical use of ascofuranone, it was found essential to discover agents which replace glycerin and exhibit an effect of the combined use in a small amount, and by using an alkaloid compound having an indole skeleton existing in a plant of the family Simaroubaceae together with ascofuranone, the prolongation of life and recovery effect in African seeping sickness was found and a patent application was filed (Japanese Patent Application No.: 2003-24643, Japanese Patent Publication A No.: 2004-23601).
Method for the preparation of fexinidazole, useful for the treatment of parasitic diseases, visceral leishmaniasis, chagas disease and human African trypanosomiasis. Family members of the product patent, WO2005037759, are expected to expire from October 2024. This to be the first application from Drugs for Neglected Diseases Initiative (DNDi) on this API. DNDi in collaboration with Sanofi, the Swiss Tropical & Public Health Institute and the University of Dundee, is developing fexinidazole, an antiparasitic agent, for treating human African trypanosomiasis (HAT) and visceral Leishmaniasis (VL). By June 2013, phase I clinical studies had been completed and at that time, DNDi was planning to initiate a phase II proof-of-concept study in VL patients in early 2013.
fexinidazole[inn], 59729-37-2, 1-Methyl-2-((4-(methylthio)phenoxy)methyl)-5-nitro-1H-imidazole, Fexinidazol, Fexinidazolum
………………..
http://www.google.com/patents/EP1681280A1?cl=en
…………..
US 4042705
http://www.google.co.in/patents/US4042705
…………
new patent june 2014
WO-2014079497
Process for preparing fexinidazole – comprising the reaction of 1-methyl-2-hydroxymethyl-5-nitro-imidazole with methanesulfonyl chloride, followed by reaction with 4-methylmercapto-phenol, and further manipulative steps.
1-Methyl-2-hydroxymethyl-5-nitro-imidazole is (I) and 1-methyl-2-(4-methylmercapto-phenyloxymethyl)-5-nitro-imidazole (fexinidazole) is (II) (claim 1, page 12).
The synthesis of (II) via intermediate (I) is described (example 1, pages 6-8).
A process for preparing fexinidazole comprising the reaction of 1-methyl-2-hydroxymethyl-5-nitro-imidazole with methanesulfonyl chloride in the presence of a suspension of powdered alkaline carbonate (eg potassium carbonate) in an anhydrous organic solvent (eg acetone), followed by reaction with 4-methylmercapto-phenol, removal of hydrochloride salt, and isolation and purification is claimed. Also claimed is their use for treating parasitic diseases, visceral leishmaniasis, chagas disease, and human African trypanosomiasis. Fexinidazole is known to be an antiparasitic agent.
|
2-1-1983
|
The activity of fexinidazole (HOE 239) against experimental infections with Trypanosoma cruzi, trichomonads and Entamoeba histolytica.
|
Annals of tropical medicine and parasitology
|
|
|
1-1-1983
|
The use of the 2 substituted 5-nitroimidazole, Fexinidazole (Hoe 239) in the treatment of chronic T. brucei infections in mice.
|
Zeitschrift für Parasitenkunde (Berlin, Germany)
|
|
5-1-2011
|
1-Aryl-4-nitro-1H-imidazoles, a new promising series for the treatment of human African trypanosomiasis.
|
European journal of medicinal chemistry
|
|
|
2-1-2011
|
Compounds containing 2-substituted imidazole ring for treatment against human African trypanosomiasis.
|
Bioorganic & medicinal chemistry letters
|
|
|
1-1-2011
|
Trypanocidal activity of nitroaromatic prodrugs: current treatments and future perspectives.
|
Current topics in medicinal chemistry
|
|
|
12-1-2010
|
Potential new drugs for human African trypanosomiasis: some progress at last.
|
Current opinion in infectious diseases
|
|
|
7-1-2010
|
Cross-resistance to nitro drugs and implications for treatment of human African trypanosomiasis.
|
Antimicrobial agents and chemotherapy
|
|
|
1-1-2010
|
Fexinidazole–a new oral nitroimidazole drug candidate entering clinical development for the treatment of sleeping sickness.
|
PLoS neglected tropical diseases
|
|
|
1-1-1999
|
[Use of megazol for the treatment of trypanosomiasis].
|
Médecine tropicale : revue du Corps de santé colonial
|
|
|
11-1-1998
|
A method to assess invasion and intracellular replication of Trypanosoma cruzi based on differential uracil incorporation.
|
Journal of immunological methods
|
|
|
10-1-1996
|
Topical chemotherapy for experimental murine African CNS-trypanosomiasis: the successful use of the arsenical, melarsoprol, combined with the 5-nitroimidazoles, fexinidazole or MK-436.
|
Tropical medicine & international health : TM & IH
|
|
|
6-1-1991
|
Chemotherapy of CNS-trypanosomiasis: the combined use of the arsenicals and nitro-compounds.
|
|
11-15-2013
|
Targeting the human parasite Leishmania donovani: discovery of a new promising anti-infectious pharmacophore in 3-nitroimidazo[1,2-a]pyridine series.
|
Bioorganic & medicinal chemistry
|
|
|
10-1-2013
|
The R enantiomer of the antitubercular drug PA-824 as a potential oral treatment for visceral Leishmaniasis.
|
Antimicrobial agents and chemotherapy
|
|
|
2-1-2013
|
Assessing the essentiality of Leishmania donovani nitroreductase and its role in nitro drug activation.
|
Antimicrobial agents and chemotherapy
|
|
|
9-1-2012
|
Genotoxicity profile of fexinidazole–a drug candidate in clinical development for human African trypanomiasis (sleeping sickness).
|
Mutagenesis
|
|
|
7-15-2012
|
Discovery of nitroheterocycles active against African trypanosomes. In vitro screening and preliminary SAR studies.
|
Bioorganic & medicinal chemistry letters
|
|
|
2-1-2012
|
The anti-trypanosome drug fexinidazole shows potential for treating visceral leishmaniasis.
|
Science translational medicine
|
|
|
1-1-2012
|
Fexinidazole: a potential new drug candidate for Chagas disease.
|
PLoS neglected tropical diseases
|
|
|
1-1-2012
|
Management of trypanosomiasis and leishmaniasis.
|
British medical bulletin
|
|
|
12-1-2011
|
Antitrypanosomal activity of fexinidazole, a new oral nitroimidazole drug candidate for treatment of sleeping sickness.
|
Antimicrobial agents and chemotherapy
|
|
|
6-1-2011
|
Development of novel drugs for human African trypanosomiasis.
|
Future microbiology
|
| US3682951 * | 2 Nov 1970 | 8 Aug 1972 | Searle & Co | 1-{8 {62 -(1-adamantyloxy)halophenethyl{9 {0 imidazoles and congeners |
| US3714179 * | 8 Sep 1970 | 30 Jan 1973 | Searle & Co | 1-alkyl-2-furfurylthioimidazoles and congeners |
| US3796704 * | 16 Aug 1971 | 12 Mar 1974 | Bayer Ag | Phenyl-imidazolylalkanyl derivatives |
| US3828065 * | 11 Dec 1972 | 6 Aug 1974 | Searle & Co | 2-methyl-5-nitro-1-(2-phenylthioethyl)imidazoles |
| US3842097 * | 22 Jan 1973 | 15 Oct 1974 | Searle & Co | 2-(phenoxyalkylthio)imidazoles and congeners |
| US3910925 * | 24 May 1974 | 7 Oct 1975 | Searle & Co | {8 2-(2-Methyl-5-nitro-1-imidazolyl)ethyl{9 benzo(b)pyridyloxy ethers |
| US3922277 * | 14 Nov 1974 | 25 Nov 1975 | Hoechst Ag | (1-Alkyl-5-nitro-imidazolyl-2-alkyl)-pyridyl compounds |
| DE2124103A1 * | 14 May 1971 | 25 Nov 1971 | Title not available |
References
- Raether, W; Seidenath, H (1983). “The activity of fexinidazole (HOE 239) against experimental infections with Trypanosoma cruzi, trichomonads and Entamoeba histolytica”. Annals of Tropical Medicine and Parasitology 77 (1): 13–26. PMID 6411009.
- Jennings, FW; Urquhart, GM (1983). “The use of the 2 substituted 5-nitroimidazole, Fexinidazole (Hoe 239) in the treatment of chronic T. brucei infections in mice”. Zeitschrift für Parasitenkunde 69 (5): 577–581. doi:10.1007/bf00926669. PMID 6636983.
- Wyllie, S; Patterson, S; Stojanovski, FRC; Norval, S; Kime, R; Read, RD; Fairlamb, AH (2012). “The anti-trypanosome drug fexinidazole shows potential for treating visceral leishmaniasis”. Science Translational Medicine 4 (119): 119re1.doi:10.1126/scitranslmed.3003326. PMC 3457684. PMID 22301556.
- Sokolova, AY; Wyllie, S; Patterson, S; Oza, SL; Read, RD; Fairlamb, AH (2010). “Cross-resistance to nitro drugs and implications for treatment of human African trypanosomiasis”. Antimicrobial Agents and Chemotherapy 54 (7): 2893–900. doi:10.1128/AAC.00332-10.PMID 20439607.
- “Jump-Start on Slow Trek to Treatment for a Disease”. New York Times. January 8, 2008.
- “Fexinidazole Progresses into Clinical Development”. DNDi Newsletter. November 2009.
- “Sanofi-aventis and DNDi enter into a Collaboration Agreement on a New Drug for Sleeping Sickness, Fexinidazole”. DNDi. May 18, 2009.
- Torreele, E; Bourdin Trunz, B; Tweats, D; Kaiser, M; Brun, R; Mazué, G; Bray, MA; Pécoul, B (2010). “Fexinidazole–a new oral nitroimidazole drug candidate entering clinical development for the treatment of sleeping sickness”. In Boelaert, Marleen. PLOS Neglected Tropical Diseases 4 (12): e923. doi:10.1371/journal.pntd.0000923. PMC 3006138. PMID 21200426.

Filed under: Phase2 drugs, Uncategorized Tagged: Fexinidazole, Hoe-239, phase 2, Trypanosomiasis