![]()
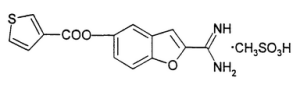
AMUVATINIB
| Name |
N-(3,4-Methylenedioxiphenylmethyl) -4 – (benzofuro [3,2-d] pyrimidin-4-yl) piperazine-1-carbothioamide. |
| CAS |
850879-09-3 |
| Formula |
C 23 H 21 N 5 O 3 S |
| MW |
447.51 |
| Synonim |
MN-470, SGI-0470-03 |
Amuvatinib (MP-470) is an orally bioavailable synthetic carbothioamide with potential antineoplastic activity. Multitargeted receptor tyrosine kinase inhibitor MP470 binds to mutant forms of the stem cell factor receptor (c-Kit; SCFR), inhibiting clinically relevant mutants of this receptor tyrosine kinase that may be associated with resistance to therapy. In addition, MP470 inhibits activities of other receptor tyrosine kinases, such as c-Met, Ret oncoprotein, and mutant forms of Flt3 and PDGFR alpha, which are frequently dysregulated in variety of tumors. This agent also suppresses the induction of DNA repair protein Rad51, thereby potentiating the activities of DNA damage-inducing agents. Mutant forms of c-Kit are often associated with tumor chemoresistance.
![]()
![]()
http://www.google.co.in/patents/EP1678166A2?cl=en
Scheme 1
![Figure imgf000034_0001]()
EXAMPLE 34 Synthesis and Analysis of Further Illustrative Compounds Compound (111-1-3), also referred to herein as HPK56/MP-470, is an illustrative compound of the present invention having the following structure:
![Figure imgf000104_0001]()
Analogues of (111-1 -3) were designed and synthesized in order to evaluate and optimize kinase selectivity, aqueous solubility, and to improve pharmacokinetic and pharmacodynamic profiles. Illustrative synthesis approaches for generating (111-1 -3) analogues are depicted in the synthesis schemes below. Synthesis of R-i substituted benzofuranopyrimidines was undertaken. The methyl 3-guanidinobenzofuran-2-carboxylate is prepared from methyl 3-aminobenzofuran-2-carboxylate by reacting with cyanoacetamide in presence of dioxane and dry HCI gas. The obtained guanidine is cyclized in the presence of aqueous NaOH. Similar procedures were utilized for preparing 2- substituted (111-1-3) and its analogues as depicted in the Schemes 8-10 set forth below. Introduction of -NH2 at the 2 position was utilized for various sulfonic, inorganic and hydroxyacid salts. Illustrative compounds are shown in Table 4 below.Table 4
Scheme 1
Scheme 2 Scheme 3
,-NH2 /N Cl S
EXAMPLE 35 Analysis of Compound Binding and Inhibitory Activity against c-kit Mutants
The published crystal structure of c-kit kinase (pdb code:1 PKG) and its mutated structure were used to study the mode of binding of compound (111-1-3) (HPK56/MP-470), a benzofuranopyrimidine compound, its 2-substituted analogs, and quinazoline derivatives.
(Ill- 1-3)
|
Information about this agent
|
According to news published on 15 Apr 2008; Research data build upon previous results showing that MP-470 exhibits anti-tumor activity in breast and http://www.medicalnewstoday.com/articles/150086.php”>prostate cancer cells. The fact that MP-470 in combination with erlotinib effectively suppressed the HER pathway suggests that concurrent administration of both compounds could represent a new treatment for prostate and breast cancers.
1. Kreidberg, Jordan A.; Qin, Shan. Method using a cMET inhibitor for the treatment of polycystic kidney disease. PCT Int. Appl. (2009), 43pp. CODEN: PIXXD2 WO 2009111529 A2 20090911 CAN 151:350826 AN 2009:1107388
2. Fujiwara, Masahiro; Fujita, Masayuki. Curing agents, adhesive curable compositions, articles and coating materials therefrom, and optical materials formed by using the compositions. Jpn. Kokai Tokkyo Koho (2008), 39pp. CODEN: JKXXAF JP 2008260894 A 20081030 CAN 149:472741 AN 2008:1303932
3. Janne, Pasi A.; Engelman, Jeffrey; Cantley, Lewis C. Methods for treating cancer resistant to ErbB therapeutics. PCT Int. Appl. (2008), 96pp. CODEN: PIXXD2 WO 2008127710 A2 20081023 CAN 149:486836 AN 2008:1282443
4. Nakagawa, Kiyoshi; Kurushima, Yoshiaki; Mizuma, Masahiro. Fabric with highly-expanded layer and process for production thereof. PCT Int. Appl. (2007), 44pp. CODEN: PIXXD2 WO 2007083641 A1 20070726 CAN 147:213021 AN 2007:815020
5. Mahadevan, D.; Cooke, L.; Riley, C.; Swart, R.; Simons, B.; Della Croce, K.; Wisner, L.; Iorio, M.; Shakalya, K.; Garewal, H.; Nagle, R.; Bearss, D. A novel tyrosine kinase switch is a mechanism of imatinib resistance in gastrointestinal stromal tumors. Oncogene (2007), 26(27), 3909-3919. CODEN: ONCNES ISSN:0950-9232. CAN 147:226484 AN 2007:613908
6. Gong, QingJie; Han, DongYu; Wang, YuRong. Experimental determination of scheelite solubility in 4.0% NaCl solution in critical region. Yanshi Xuebao (2006), 22(12), 3052-3058. CODEN: YANXEU ISSN:1000-0569. CAN 147:34728 AN 2007:301330
7. Tokunaga, Koji; Mukai, Takashi; Kishigami, Akira. Two-component weak solvent-based coating compositions with no curing interference by dewing. Jpn. Kokai Tokkyo Koho (2005), 15 pp. CODEN: JKXXAF JP 2005239815 A 20050908 CAN 143:249850 AN 2005:979155
8. Lauer, S. J.; Shuster, J. J.; Mahoney, D. H., Jr.; Winick, N.; Toledano, S.; Munoz, L.; Kiefer, G.; Pullen, J. D.; Steuber, C. P.; Camitta, B. M. A comparison of early intensive methotrexate/mercaptopurine with early intensive alternating combination chemotherapy for high-risk B-precursor acute lymphoblastic leukemia: A pediatric oncology group phase III randomized trial. Leukemia (2001), 15(7), 1038-1045. CODEN: LEUKED ISSN:0887-6924. CAN 135:338846 AN 2001:586045
9. Sen, Ayusman; Hennis, April. Palladium (II) catalyzed polymerization of norbornene and acrylates. PCT Int. Appl. (2001), 22 pp. CODEN: PIXXD2 WO 2001021670 A1 20010329 CAN 134:252774 AN 2001:228935
10. Patel, Raman; Mallin, Dan; Saunders, Keith; Tiberio, Patrick; Andries, John. Polymer compositions containing polyolefins, polar polymers and block or graft copolymer compatibilizers and their preparation. PCT Int. Appl. (1999), 25 pp. CODEN: PIXXD2 WO 9950350 A1 19991007 CAN 131:272658 AN 1999:640932
11. Koehler, Burkhard; Imai, Seisaku; Doering, Joachim; Ruesseler, Wolfgang; Dorf, Ernst Ullrich. Mixtures of polyarylene sulfides, glass fibers, and polymaleimides with good mechanical properties. Ger. Offen. (1992), 4 pp. CODEN: GWXXBX DE 4105913 A1 19920827 CAN 118:82122 AN 1993:82122
12. Itoh, Michiya; Fuke, Kiyokazu; Kobayashi, Sachiko. Direct observation of intramolecular anthracene excimer in 1,3-dianthrylpropane. Journal of Chemical Physics (1980), 72(2), 1417-18. CODEN: JCPSA6 ISSN:0021-9606. CAN 92:155340 AN 1980:155340
13. Tomillero A; Moral M A Gateways to clinical trials. Methods and findings in experimental and clinical pharmacology (2009), 31(9), 597-633. Journal code: 7909595. ISSN:0379-0355. PubMed ID 20094643 AN 2010048694
14. Tomillero A; Moral M A Gateways to clinical trials. Methods and findings in experimental and clinical pharmacology (2009), 31(8), 541-57. Journal code: 7909595. ISSN:0379-0355. PubMed ID 19967103 AN 2009808578
15. Gorrand Jean-Marie; Doly Michel; Bacin Franck Macular pigment density assessed by directional fundus reflectance. Journal of the Optical Society of America. A, Optics, image science, and vision (2009), 26(8), 1847-54. Journal code: 9800943. ISSN:1084-7529. PubMed ID 19649122 AN 2009529589
16. Tomillero A; Moral M A Gateways to clinical trials. Methods and findings in experimental and clinical pharmacology (2009), 31(4), 263-98. Journal code: 7909595. ISSN:0379-0355. PubMed ID 19557204 AN 2009445725
Filed under:
Uncategorized Tagged:
AMUVATINIB ![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()




















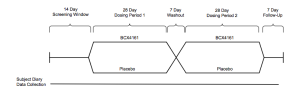
















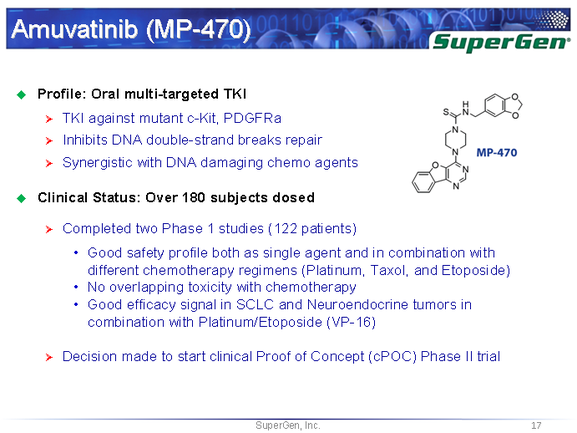












 Source:
Source: 





