![img]()
![2D chemical structure of 1443763-60-7]()
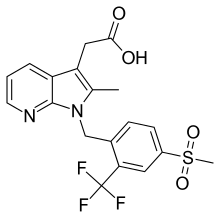
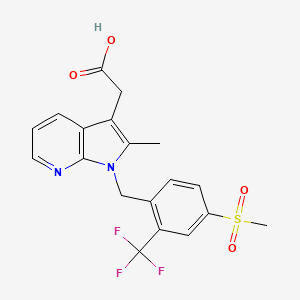
Empesertib , BAY 1161909, Mps1-IN-5, (-)-BAY-1161909
CAS 1443763-60-7
Chemical Formula: C29H26FN5O4S
Molecular Weight: 559.6164
UNII-02Y3Z2756M
(αR)-4-Fluoro-N-[4-[2-[[2-methoxy-4-(methylsulfonyl)phenyl]amino][1,2,4]triazolo[1,5-a]pyridin-6-yl]phenyl]-α-methylbenzeneacetamide
(R)-2-(4-fluorophenyl)-N-(4-(2-((2-methoxy-4-(methylsulfonyl)phenyl)amino)-[1,2,4]triazolo[1,5-a]pyridin-6-yl)phenyl)propanamide
(2R)-2-(4-Fluorophenyl)-N-(4-(2-((4-(methanesulfonyl)-2-methoxyphenyl)amino)(1,2,4)triazolo(1,5-a)pyridin-6-yl)phenyl)propanamide
Benzeneacetamide, 4-fluoro-N-(4-(2-((2-methoxy-4-(methylsulfonyl)phenyl)amino)(1,2,4)triazolo(1,5-a)pyridin-6-yl)phenyl)-alpha-methyl-, (alphaR)-
PHASE 1, BAYER, CANCER
PRODUCT PATENT, WO2013087579, https://www.google.com/patents/WO2013087579A1
![Image result]()
SYNTHESIS, COMING WATCH OUT THIS SPACE SOON
Empesertib, also known as BAY1161909, is an orally bioavailable, selective inhibitor of the serine/threonine monopolar spindle 1 (Mps1) kinase, with potential antineoplastic activity. Upon administration, the Mps1 kinase inhibitor BAY1161909 binds to and inhibits the activity of Mps1. This causes inactivation of the spindle assembly checkpoint (SAC), accelerated mitosis, chromosomal misalignment, chromosomal missegregation, mitotic checkpoint complex destabilization, and increased aneuploidy. This leads to the induction of cell death in cancer cells overexpressing Mps1.
BAY-1161909 is an oral dual specificity protein kinase TTK inhibitor in early clinical trials at Bayer for the treatment of advanced malignancies in combination with paclitaxel.
Bayer and INSERM are developing BAY-1161909 , presumed to be the lead from monopolar spindle-1 inhibitors, including Mps-BAY-2b and Mps-BAY-2c, for the oral treatment of cancer; in July 2016, BAY-1161909 was reported to be in phase I clinical trial.
Mps-1 (Monopolar Spindle 1 ) kinase (also known as Tyrosine Threonine Kinase, TTK). Mps-1 is a dual specificity Ser/Thr kinase which plays a key role in the activation of the mitotic checkpoint (also known as spindle checkpoint, spindle assembly checkpoint) thereby ensuring proper chromosome segregation during mitosis [Abrieu A et al., Cell, 2001 , 106, 83-93]. Every dividing cell has to ensure equal separation of the replicated chromosomes into the two daughter cells. Upon entry into mitosis, chromosomes are attached at their kinetochores to the microtubules of the spindle apparatus. The mitotic checkpoint is a surveillance mechanism that is active as long as unattached kinetochores are present and prevents mitotic cells from entering anaphase and thereby completing cell division with unattached chromosomes [Suijkerbuijk SJ and Kops GJ, Biochemica et Biophysica Acta, 2008, 1786, 24- 31 ; Musacchio A and Salmon ED, Nat Rev Mol Cell Biol., 2007, 8, 379-93]. Once all kinetochores are attached in a correct amphitelic, i.e. bipolar, fashion with the mitotic spindle, the checkpoint is satisfied and the cell enters anaphase and proceeds through mitosis. The mitotic checkpoint consists of a complex network of a number of essential proteins, including members of the MAD (mitotic arrest deficient, MAD 1 -3) and Bub (Budding uninhibited by benzimidazole, Bub 1 -3) families, the motor protein CENP-E, Mps-1 kinase as well as other components, many of these being over-expressed in proliferating cells (e.g. cancer cells) and tissues [Yuan B et al., Clinical Cancer Research, 2006, 12, 405-10]. The essential role of Mps-1 kinase activity in mitotic checkpoint signalling has been shown by shRNA-silencing, chemical genetics as well as chemical inhibitors of Mps-1 kinase [Jelluma N et al., PLos ONE, 2008, 3, e2415; Jones MH et al., Current Biology, 2005, 15, 160-65; Dorer RK et al., Current Biology, 2005, 15, 1070-76; Schmidt M et al., EMBO Reports, 2005, 6, 866-72].
There is ample evidence linking reduced but incomplete mitotic checkpoint function with aneuploidy and tumorigenesis [Weaver BA and Cleveland DW, Cancer Research, 2007, 67, 10103-5; King RW, Biochimica et Biophysica Acta, 2008, 1786, 4-14]. In contrast, complete inhibition of the mitotic checkpoint has been recognised to result in severe chromosome missegregation and induction of apoptosis in tumour cells [Kops GJ et al., Nature Reviews Cancer, 2005, 5, 773-85; Schmidt M and Medema RH, Cell Cycle, 2006, 5, 159-63; Schmidt M and Bastians H, Drug Resistance Updates, 2007, 10, 162-81]. Therefore, mitotic checkpoint abrogation through pharmacological inhibition of Mps-1 kinase or other components of the mitotic checkpoint represents a new approach for the treatment of proliferative disorders including solid tumours such as carcinomas and sarcomas and leukaemias and lymphoid malignancies or other disorders associated with uncontrolled cellular proliferation.
Different compounds have been disclosed in prior art which show an inhibitory effect on Mps-1 kinase:
WO 2009/024824 A1 discloses 2-Anilinopurin-8-ones as inhibitors of Mps-1 for the treatment of proliferate disorders. WO 2010/124826 A1 discloses substituted imidazoquinoxaline compounds as inhibitors of Mps-1 kinase. WO 2011 /026579 A1 discloses substituted aminoquinoxalines as Mps-1 inhibitors.
Substituted triazolopyndine compounds have been disclosed for the treatment or prophylaxis of different diseases:
WO 2008/025821 A1 (Cellzome (UK) Ltd) relates to triazole derivatives as kinase inhibitors, especially inhibitors of ITK or PI3K, for the treatment or prophylaxis of immunological, inflammatory or allergic disorders. Said triazole derivatives are exemplified as possessing an amide, urea or aliphatic amine substituent in position 2.
WO 2009/047514 A1 (Cancer Research Technology Limited) relates to [1 ,2,4]- triazolo-[1 ,5-a]-pyridine and [1 ,2,4]-triazolo-[1 ,5-c]-pyrimidine compounds which inhibit AXL receptor tyrosine kinase function, and to the treatment of diseases and conditions that are mediated by AXL receptor tyrosine kinase, that are ameliorated by the inhibition of AXL receptor tyrosine kinase function etc., including proliferative conditions such as cancer, etc.. Said compounds are exemplified as possessing a substituent in the 5-position and a substituent in the 2-position.
WO 2009/010530 A1 discloses bicyclic heterorayl compounds and their use as phosphatidyli nositol (PI) 3-kinase. Among other compounds also substituted triazolopyridines are mentioned.
WO 2009/027283 A1 discloses triazolopyridine compounds and their use as ASK (apoptosis signal-regulating kinase) inhibitors for the treatment of autoimmune diseases and neurodegenerative diseases. WO 2010/092041 A1 (Fovea Pharmaceuticals SA) relates to [1 ,2,4]-triazolo- [1 ,5-a] -pyridines, which are said to be useful as selective kinase inhibitors, to methods for producing such compounds and methods for treating or ameliorating kinase-mediated disorder. Said triazole derivatives are exemplified as possessing a 2-chloro-5-hydroxyphenyl substituent in the 6- position of the [1 ,2,4]-triazolo-[1 ,5-a]-pyridine.
WO 2011 /064328 A1 , WO 2011 /063907 A1 , and WO 2011 /063908 A1 (Bayer Pharma AG) relate to [1 ,2,4]-triazolo-[1 ,5-a]-pyridines and their use for inhibition of Mps-1 kinase.
WO 2011 /064328 A1 discloses com ounds of fomula S2:
S2
in which
R1 is an aryl- or heteroaryl- group; wherein the aryl- or heteroaryl- group can be substituted inter alia with -N(H)C(=0)R6 or -C(=0)N(H)R6 ; in which R6represents a hydrogen or a Ci-C6-alkyl- group; the Ci-C6-alkyl- group optionally being substituted with halo-, hydroxyl-, d-C3-alkyl, R70-. WO 2011 /064328 A1 does not disclose compounds of the present invention as defined below.
WO 2011 /063907 A1 discloses compounds of fomula S1 :
S1
in which
R1 is an aryl group which is substituted at least one time; whereas the at least one substituent inter alia can be -N(H)C(=0)R6 or -C(=0)N(H)R6 ; in which R6represents a group selected from C3-C6-cycloalkyl, 3- to 10-membered heterocyclyl-, aryl-, heteroaryl-, -(CH2)q-(C3-C6-cycloalkyl), -(CH2)q-(3- to 10- membered heterocyclyl), -(CH2)q-aryl, or -(CH2)q-heteroaryl, wherein R6 is optionally substituted, and q is 0, 1 , 2 or 3;
R2 represents a substituted or unsubstituted aryl- or heteroaryl- group;
R3 and R4 inter alia can be hydrogen; and
R5 represents a substituted or unsubstituted Ci-C6-alkyl group.
WO 2011 /063908 A1 discloses com ounds of fomula S3:
S3
in which
R1 is an aryl group which is substituted at least one time; whereas the at least one substituent inter alia can be -N(H)C(=0)R6 or -C(=0)N(H)R6 ; in which R6inter alia represents a group selected from C3-C6-cycloalkyl, 3- to 10- membered heterocyclyl-, aryl-, heteroaryl-, -(CH2)q-(C3-C6-cycloalkyl), -(CH2)q– (3- to 10-membered heterocyclyl), -(CH2)q-aryl, and -(CH2)q-heteroaryl, wherein R6 is optionally substituted, and q is 0, 1 , 2 or 3;
R2 represents a substituted or unsubstituted aryl- or heteroaryl- group;
R3 and R4 inter alia can be hydrogen; and
R5 is hydrogen.
There are patent applications which are related to [1 ,2,4]-triazolo-[1 ,5-a]- pyridines and their use for inhibition of Mps-1 kinase, but which have not been published at the time of filing of this patent application: Subject matter of the EP patent applications No. 11167872.8, and No. 11167139.2 as well as of the patent application PCT/EP2011 /059806 are com ounds of fomula S4:
S4
in which
R1 represents inter alia a phenyl- group which is substituted at least one time; whereas the at least one substituent inter alia can be -N(H)C(=0)R6; in which R6inter alia can be -(CH2)q-aryl, wherein R6 is optionally substituted, and q is 0, 1 , 2 or 3;
R2 represents a substituted or unsubstituted aryl- or heteroaryl- group;
R3 and R4 inter alia can be hydrogen; and
R5 is hydrogen. However, the state of the art described above does not specifically disclose the substituted triazolopyridine compounds of general formula (I) of the present invention, or a tautomer, an N-oxide, a hydrate, a solvate, or a salt thereof, or a mixture of same, as described and defined herein, and as hereinafter referred to as “compounds of the present invention”, or their pharmacological activity.
The above mentioned patent applications which are related to [1 ,2,4]-triazolo- [1 ,5-a] -pyridines mainly focus on the effectiveness of the compounds in inhibiting Mps-1 kinase, expressed by the half maximal inhibitory concentration (IC50) of the compounds. For example, in WO 2011 /063908 A1 the effectiveness in inhibiting Mps-1 kinase was measured in an Mps-1 kinase assay with a concentration of 10 μΜ adenosine triphosphate (ATP).
The cellular concentration of ATP in mammals is in the millimolar range. Therefore it is important that a drug substance is also effective in inhibiting Mps-1 kinase in a kinase assay with a concentration of ATP in the millimolar range, e.g. 2 mM ATP, in order to potentially achieve an antiproliferative effect in a cellular assay. In addition, as one of ordinary skill in the art knows, there a many more factors determining the druglikeness of a compound. The objective of a preclinical development is to assess e.g. safety, toxicity, pharmacokinetics and metabolism parameters prior to human clinical trials. One important factor for assessing the druglikeness of a compound is the metabolic stability. The metabolic stability of a compound can be determined e.g. by incubating the compound with a suspension of liver microsomes from e.g. a rat, a dog and/or a human (for details see experimental section). Another important factor for assessing the druglikeness of a compound for the treatment of cancer is the inhibition of cell proliferation which can be determined e.g. in a HeLa cell proliferation assay (for details see experimental section). Surprisingly it was found, that the compounds of the present invention are characterized by :
– an IC50 lower than or equal to 1 nM (more potent than 1 nM) in an Mps-1 kinase assay with a concentration of 10 μΜ ATP, and
– an IC50 lower than 10 nM (more potent than 10 nM) in an Mps-1 kinase assay with a concentration of 2 mM ATP, and – a maximum oral bioavailability (Fmax) in rat that is higher than 50 % determined by means of rat liver microsomes as described below, and
– a maximum oral bioavailability (Fmax) in dog that is higher than 45 % determined by means of dog liver microsomes as described below, and
– a maximum oral bioavailability (Fmax) in human that is higher than 45 %, determined by means of human liver microsomes as described below, and
– an IC50 lower than 600 nM in a HeLa cell proliferation assay as described below. Hence, the compounds of the present invention have surprising and advantageous properties. These unexpected findings give rise to the present selection invention. The compounds of the present invention are purposively selected from the above mentioned prior art due to their superior properties. In particular, said compounds of the present invention may therefore be used for the treatment or prophylaxis of diseases of uncontrolled cell growth, proliferation and/or survival, inappropriate cellular immune responses, or inappropriate cellular inflammatory responses or diseases which are accompanied with uncontrolled cell growth, proliferation and/or survival, inappropriate cellular immune responses, or inappropriate cellular inflammatory responses, particularly in which the uncontrolled cell growth, proliferation and/or survival, inappropriate cellular immune responses, or inappropriate cellular inflammatory responses is mediated by Mps-1 kinase, such as, for example, haemotological tumours, solid tumours, and/or metastases thereof, e.g. leukaemias and myelodysplastic syndrome, malignant lymphomas, head and neck tumours including brain tumours and brain metastases, tumours of the thorax including non-small cell and small cell lung tumours, gastrointestinal tumours, endocrine tumours, mammary and other gynaecological tumours, urological tumours including renal, bladder and prostate tumours, skin tumours, and sarcomas, and/or metastases thereof.
PATENT
WO2013087579
https://www.google.com/patents/WO2013087579A1
Synthesis of Examples
Compounds of the present invention
Example01.01
(2 ?)-2-(4-fluorophenyl)-N-[4-(2-{[2-methoxy-4-(methylsulfonyl)phenyl]- amino}[1,2,4]triazolo[1,5-a]pyridin-6-yl)phenyl]propanamide
![Figure imgf000139_0001]()
To a stirred suspension of Int08.011 (6.0 g) in DMF (48 mL) and dichloromethane (96 mL) was added sodium bicarbonate (3.69 g), (2/?)-2-(4- fluorophenyl)propanoic acid (2.71 g) and HATU (8.36 g). The mixture was stirred at room temperature for 4 h. Water was added, and the mixture was stirred for 30 minutes. A half-saturated solution of sodium bicarbonate was added and the mixture was extracted with ethyl acetate. The organic phase was washed with saturated sodium chloride solution, dried (sodium sulfate) and the solvent was removed in vacuum. Silicagel chromatography gave a solid that was triturated with ethyl acetate to give 7,44 g of the title compound. 1H-NMR (400MHz, DMSO-d6): δ [ppm]= 1.40 (d, 3H), 3.16 (s, 3H), 3.84 (q, 1H), 3.96 (s, 3H), 7.09 – 7.18 (m, 2H), 7.36 – 7.44 (m, 3H), 7.51 (dd, 1H), 7.63 – 7.76 (m, 5H), 7.92 (dd, 1H), 8.48 (d, 1H), 8.60 (s, 1H), 9.10 (d, 1 H), 10.16 (s, 1H).
[a]D20 : -77.0° (in DMSO).
Column: Chiralcel OD-RH 150×4.6; Flow: 1.00 mL/min; Solvent: A: Water with 0.1 % formic acid, B: Acetonitrile; Solvent mixture: 40% A + 60% B. Run Time: 30 min. Retention Time: 12.83 min; UV 254 nm; Enantiomeric Ratio: <1% : > 99%. Racemate01.01.r
Starting with Int01.05 and Int03.02, Racemate01.01.r was prepared analogously to the procedure for the preparation of Int08.020.
Racemate01.02.r
PATENT
WO2014009219
PATENT
WO-2017216025
Novel crystalline polymorphic forms of (2R)-2-(4-fluorophenyl)-N-[4-(2-{[2- methoxy-4-(methylsulfonyl)phenyl]amino}[1,2,4]triazolo[1,5-a]pyridin-6-yl)phenyl]propan-amide 4-toluenesulfonate (empesertib), and crystalline (2R)-2-(4-fluorophenyl)-N-[4-(2-{[2-methoxy-4- (methylsulfonyl)phenyl]amino}[1,2,4]triazolo[1,5-a]pyridin-6-yl)phenyl]propanamide 4- toluenesulfonate monohydrate, composition comprising them and their preparation methods are claimed. Also claims their use for treating various cancers. It is disclosed that empesertib is a potent Mps-1 kinase inhibitor.
MPS-1 INHIBITORS
The present invention covers crystalline, anhydrous (2/?)-2-(4-fluorophenyl)-/V-[4-(2-{[2-methoxy^-imethylsulfonyljphenyllaminojll^^ltriazololl^-olpyridin-e-yljphenyllpropan-amide 4-toluenesulfonate, and crystalline (2/?)-2-(4-fluorophenyl)-/V-[4-(2-{[2-methoxy-4-(methylsulfonyl)phenyl]amino}[l,2,4]triazolo[l,5-a]pyridin-6-yl)phenyl]propanamide 4-toluenesulfonate monohydrate, as compounds per se, a method of preparing said crystalline, anhydrous compound, pharmaceutical compsitions and pharmaceutical combinations comprising said crystalline, anhydrous compound, and uses of said crystalline, anhydrous compound in the treatment or prophylaxis of cancer, in particular pancreatic cancer, glioblastoma, ovarian cancer, non-small cell lung carcinoma, breast cancer, and/or gastric cancer.
BACKGROUND OF THE INVENTION
(2R)-2-(4-fluorophenyl)-/V-[4-(2-{[2-methoxy-4-(methylsulfonyl)phenyl]-amino}[l,2,4]triazolo[l,5-a]pyridin-6-yl)phenyl]propanamide is known to be a very potent inhibitor of Mps-1 kinase.
WO 2013/087579 Al discloses the compound, data showing its pharmaceutical activity, and a method for the preparation of (2/?)-2-(4-fluorophenyl)-/V-[4-(2-{[2-methoxy-4-(methylsulfonyl)phenyl]amino}[l,2,4]triazolo[l,5-a]pyridin-6-yl)phenyl]propanamide.
WO 2014/009219 Al discloses an improved method for the preparation of (2/?)-2-(4-fluorophenyl)-/V-[4-(2-{[2-methoxy-4-(methylsulfonyl)phenyl]amino}[l,2,4]triazolo[l,5-a]pyridin-6-yl)phenyl]propan-amide.
WO 2014/195408 Al discloses pharmaceutical compositions comprising (2 ?)-2-(4-fluorophenyl)-/V-[4-(2-{[2-methoxy-4-(methylsulfonyl)phenyl]amino}[l,2,4]triazolo[l,5-a]pyridin-6-yl)phenyl]propan-amide mainly in amorphous form.
Surprisingly and unexpectedly, it was observed that a crystalline form of the anhydrous 4-toluenesulfonate salt of (2/?)-2-(4-fluorophenyl)-/V-[4-(2-{[2-methoxy-4-(methylsulfonyl)-phenyl]amino}[l,2,4]triazolo[l,5-o]pyridin-6-yl)phenyl]propanamide shows superior properties in terms of its pharmacological usability compared to the free base (2/?)-2-(4-fluorophenyl)-/V-[4-(2-{[2-methoxy-4-(methylsulfonyl)phenyl]amino}[l,2,4]triazolo[l,5-a]pyridin-6-yl)phenyl]propanamide or other salts thereof.
In accordance with a first aspect, the present invention thus covers crystalline, anhydrous (2/?)-2-(4-fluorophenyl)-/V-[4-(2-{[2-methoxy-4-(methylsulfonyl)phenyl]-amino}[l,2,4]triazolo[l,5-a]pyridin-6-yl)phenyl]propanamide 4-toluenesulfonate, of formula (I) :
![]()
(I),
hereinafter also referred to as the “anhydrous tosylate salt” or “anhydrous 4-toluenesulfonate salt”,
Example 1 : Preparation of crystalline, anhydrous (2/?)-2-(4-fluorophenyl)-/V-[4-(2-{[2-methoxy-4-(methylsulfonyl)phenvnamino}fl,2,41triazolofl,5-a1pyridin-6-vDphenyllpropanamide 4-toluene-sulfonate : method 1 (without seeding)
![]()
Without seeding:
10 g (17.9 mmol) of (2/?)-2-(4-fluorophenyl)-/V-[4-(2-{[2-methoxy-4-(methylsulfonyl)phenyl]-amino}[l,2,4]triazolo[l,5-a]pyridin-6-yl)phenyl]propanamide were suspended in 2-butanone (100 ml) and heated to 65°C 4.08 g (21.4 mmol) 4-toluenesulfonic acid monohydrate in 2-butanone (20 ml) were added at 65°C. The suspension dissolved and the product precipitated from solution. The mixture was stirred for 21h at 65°C. The mixture was cooled to 20°C over 2h. After 2h stirring at 20°C the precipitate was isolated by suction filtration and washed two times with 100 ml 2-butanone (each). The product was dried in vacuum (approximately 60 mbar) at 50°C for 7h. 12.4 g (95 % of theory) were isolated.
Example 3 : Preparation of crystalline (2/?)-2-(4-fluorophenyl)-/V-[4-(2-{[2-methoxy-4- (methylsulfonyl)phenyllannino}[l,2,41triazolo[l,5-alpyriclin-6-yl)phenyllpropanamide 4; toluene-sulfonate monohydrate: method 1 : without seeding
![]()
10 g (17.9 mmol) of (2/?)-2-(4-fluorophenyl)-W-[4-(2-{[2-methoxy-4-(methylsulfonyl)phenyl]-amino}[l,2,4]triazolo[l,5-a]pyridin-6-yl)phenyl]propanamide were suspended in 2-butanone (100 ml) and water (2.4 ml) and heated to 65°C. 4.08 g (21.4 mmol) 4-toluenesulfonic acid monohydrate in 2-butanone (20 ml) were added at 65°C. The suspension dissolved and the product precipitated from solution. The mixture was stirred for 21h at 65°C and then cooled to 20°C within 2h. After 2h stirring at 20°C the precipitate was isolated by suction filtration and washed two times with 100 ml 2-butanone (each). The product was dried in vacuum (approximately 60 mbar) at 50°C for 7h. 11.4 g (85 % of theory) were isolated.
Thermogravimetry showed a wheight loss of 2.2 weight-% while heating from 32.6°C to 100°C.
lH-N MR(DMSO-d6): δ = 1.43 (3H), 2.29 (3H), 3.20 (3H), 3.87 (1H), 4.00 (3H), 4.40-4.95 (broad signal, water) 7.09-7.21 (4H), 7.41-7.50 (5H), 7.55 (1H), 7.69-7.78 (5H), 7.97 (1H), 8.51 (1H), 8.67 (1H), 9.15 (1H), 10.21 (1H) ppm.
REFERENCES
1. Combinations for the treatment of cancer comprising a Mps-1 kinase inhibitor and a mitotic inhibitor
By Wengner, Antje Margret; Siemeister, Gerhard
From PCT Int. Appl. (2014), WO 2014198645 A1 20141218.
2. Preparation of prodrug derivatives of substituted triazolopyridine monopolar spindle 1 kinase inhibitors and their use for the treatment of cancer
By Schulze, Volker; Lerchen, Hans-Georg; Bierer, Donald; Wengner, Antje Margret; Siemeister, Gerhard; Lienau, Philip; Krenz, Ursula; Kosemund, Dirk; Stoeckigt, Detlef; Bruening, Michael; et al
From PCT Int. Appl. (2014), WO 2014198647 A2 20141218.
3. Pharmaceutical compositions comprising substituted triazolopyridine compds.
By Schulze, Volker; Bruening, Michael; Stoeckigt, Detlef
From PCT Int. Appl. (2014), WO 2014195408 A1 20141211.
4. Combinations comprising inhibitors of Mps-1 kinase and anti-apoptotic protein of the Bcl-2 family for the treatment of cancer
By Siemeister, Gerhard; Bader, Benjamin; Wengner, Antje; Mumberg, Dominik; Schulze, Volker; Kroemer, Guido; Vitale, Ilio; Jemaa, Mohamed
From PCT Int. Appl. (2014), WO 2014020043 A1 20140206.
5. Method for preparing substituted triazolopyridines as Mps-1 kinase inhibitors
By Schulze, Volker; Mais, Franz-Josef
From PCT Int. Appl. (2014), WO 2014009219 A1 20140116.
6. Preparation of triazolopyridine derivatives for use as TTK inhibitors
By Schulze, Volker; Kosemund, Dirk; Wengner, Antje Margret; Siemeister, Gerhard; Stoeckigt, Detlef; Bruening, Michael
From PCT Int. Appl. (2013), WO 2013087579 A1 20130620.
///////////BAY1161909, BAY-1161909, BAY 1161909, Empesertib, Mps1-IN-5, (-)-BAY-1161909, PHASE 1
O=C(NC1=CC=C(C2=CN3C(C=C2)=NC(NC4=CC=C(S(=O)(C)=O)C=C4OC)=N3)C=C1)[C@H](C)C5=CC=C(F)C=C5
Filed under:
PHASE 1,
PHASE1 Tagged:
BAY-1161909,
BAY1161909,
Empesertib,
Mps1-IN-5,
PHASE 1 ![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()








































 To promote all-natural methods to raise your pet in a healthy manner.
To promote all-natural methods to raise your pet in a healthy manner.