EPEREZOLID
pfizer.originator
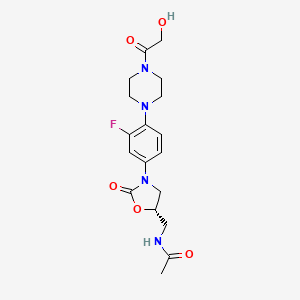
(S)-N-[[3-[3-Fluoro-4-[4-(2-hydroxyacetyl)piperazin-1-yl]phenyl]-2-oxo-1,3-oxazolidin-5-yl]methyl]acetamide
(S)-N-[[3-[3-fluoro-4-[4-(hydroxyacetyl)-l-piperazinyl]- phenyl]-2-oxo-5-oxazolidinyl]methyl]acetamide

Oxazolidinones are a new class of Gram-positive antibacterial agents which are known to those skilled in the art, see for example US 5,688,792. (S)-N-[[3-[3- fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]acetamide, known as linezolid, the compound of Example 5 of US Patent 5,688,792 is known and has the following chemical formula:
(S)-N-[[3-[3-fluoro-4-[4-(hydroxyacetyl)-l-piperazinyl]-phenyl]-2-oxo-5- oxazolidinyl]methyl]acetamide, known as eperezolid, the compound of
Example 8 of US Patent 5,837,870 is known and has the following chemical formula:
Linezolid and eperezolid can be produced by the processes set forth in US Patents 5,688,791 and 5,837,870 as well as that of International Publication WO99/24393. It is preferably produced by the process of US Patent 5,837,870.
It is preferred that the linezolid produced be used in crystal form π, which has the characteristics set forth in CHART A. Once linezolid is synthesized, crystal Form π is prepared by starting with linezolid of high enantiomeric purity. It is preferred that the linezolid be more than 98% enantiomerically pure, it is more preferred that the linezolid be more than 99% pure and it is even more preferred that the linezolid be 99.5% pure. The linezolid of greater than 98% enantiomeric purity to be used to form crystal form II can either be in solution or be a solid. The linezolid starting material, solid or solution, is mixed with a solvent selected from the group consisting of compounds of the formula: water, acetonitrile, chloroform, methylene chloride, R OH where R\ is Cι-C6 alkyl; Rι-CO-R2 where R2 is Cι-C alkyl and Ri is as defined above; phenyl substituted with 1 thru 3 Ri where Ri is as defined above; Rι-CO-O-R2 where Ri is -C alkyl and Ri is as defined above; Rι-O-R2 where
is Cι-C6 alkyl and Ri is as defined above. It is preferred that the solvent be selected from the group consisting of water, ethyl acetate, methanol, ethanol, propanol, isopropanol, butanol, acetonitrile, acetone, methyl ethyl ketone, chloroform, methylene chloride, toluene, xylene, diethyl ether, or methyl-t-butyl ether. It is more preferred that the solvent be ethyl acetate, acetone, acetonitrile, propanol, or isopropanol. It is most preferred that the solvent be ethyl acetate. The mixture of linezolid in the solvent is agitated at a temperature below 80° until crystals of Form II are formed and crystals of other solid forms, such as Form I, disappear. It is preferred to dissolve the linezolid in ethyl acetate at a temperature near the boiling point of the solvent. This mixture is cooled to a temperature of about 70°. The mixture may be seeded with crystals of Form II to facilitate crystallization. It is preferred that the solid product is cooled and agitated at a temperature between about 45° and about 60° until the solids consist only of Form II crystals. It is most preferred to maintain the slurry at a temperature of about 55°. It is preferred to mix the linezolid and solvent for at least 10 min, it is even more preferred to mix the linezolid and solvent for at least 20 min and it is most preferred to mix the linezolid and solvent for at least 30 min. The time and temperature will vary depending on the solvent selected. With ethyl acetate it is preferred to mix for not less that 60 minutes. The crystalline slurry may be further cooled to improve yield, and the solid Form II product may be isolated. The mixture may be further cooled and agitated. Other measures which can be used to facilitate crystallization include, but are not limited to, cooling, concentration of the solution by evaporation or distillation, or through addition of other solvents. The crystals are isolated by procedures known to those skilled in the art.
It is well known to those skilled in the art that the oxazolidinones are useful as anti-bacterial agents especially against Gram-positive organisms. US Patent 5,688,792 discloses that oxazolidinones can be administered IV. The preferred formulation for linezolid IV solution is: Linezolid 2.0 mg mL
Sodium Citrate Dihydrate (USP) 1.64 mg/mL
Citric Acid Anhydrous (USP) 0.85 mg/mL
Dextrose Monohydrate (USP) 50.24 mg/mL
Hydrochloric Acid ( 10%) q.s. to pH 4.8 (pH 4.6 to 5.0) Sodium hydroxide (10%) q.s. to pH 4.8 (pH 4.6 to 5.0)
Water for Injection (USP) q.s. ad 1.0 mL
The linezolid IV solution is formulated by heating water for injection from about 50 to about 65°. Next the sodium citrate, citric acid and dextrose are added and stirred until dissolved. An aqueous slurry of linezolid is added to the previous mixture and stirred until dissolved. The mixture is cooled to 25° with stirring. The pH is measured and adjusted if necessary. Last the mixture is brought to volume, if necessary, with water for injection. The mixture is filtered, filled into infusion containers, over wrapped and terminally moist heat sterilized.
The aqueous solution for IV administration can be placed in the container which is selected from the group consisting of a bag, a bottle, a vial, a large volume parenteral, a small volume parenteral, a prefilled syringe and a cassette. It is realized that a vial is a bottle. However, those skilled in the art use the term “bottle” to refers to larger bottles and “vials” to refer to smaller bottles. It is preferred that the container be a bag, a bottle, a vial or a prefilled syringe. It is more preferred that the container be a bag or bottle. It is most preferred that the container be a bag. The shape and/or size of the container is unimportant. It is preferred that the container be a bag sufficient to hold 25 to 2,000 mL of IV solution. It is preferred that the linezolid mixture be put in bags in amounts of 100, 200 or 300 mL of solution however smaller or larger volumes are acceptable.

……………..
http://www.google.com/patents/WO2007138381A2?cl=en
. Scheme 2. Synthesis of eperezolid
RR==IH-
s
Et3N,
17 (eperezolid)
1-(2-Fluoro-4-nitrophenyl)piperazine (8). To 3,4-difluoronitrobenzene (20.5 g, 129 mmol) in acetonitrile (290 mL) was added triethylamine (36 mL) and piperazine (32 g, 387 mmol). The mixture was stirred at reflux for 18 h, after which it was cooled to room temperature and partitioned between H2O (500 mL) and EtOAc (400 mL). The layers were separated and the aqueous layer was extracted with EtOAc (2 x 300 mL). The organic layers were combined and washed with saturated NaCI solution (400 mL). The saturated NaCI layer was extracted again with EtOAc (2 x 200 mL). The organic layers were combined, dried over Na2SO4, filtered and concentrated to yield 8 as a yellow solid (29 g, quant.). 1H NMR (400 MHz, CDCI3) δ 1.63 (s, 1 H), 3.04-3.06 (m, 4H), 3.25-3.28 (m, 4H), 6.91 (t, J=8.7, 1 H), 7.90 (dd, J=13.2, 2.5, 1 H), 7.97- 8.00 (m, 1H).
3-Fluoro-4-(piperazin-1-yl)benzenamine (9). Compound 8 (10.0 g, 44.4 mmol) was dissolved in anhydrous EtOH (222 mL) and placed in a Parr pressure flask. PtO2 catalyst (31 mg) was added and the mixture was agitated under 50-60 psi of H2 on a Parr apparatus for 30 min, after which the reaction mixture was vented, more catalyst was added (78 mg) and the reaction mixture was submitted to 50-60 psi of H2 for another 30 min. The reaction mixture was filtered on Celite, the solid was washed with MeOH1 and the combined filtrates were concentrated to give 9 as a yellow solid (8.7 g, quant.). 1H NMR (400 MHz, CDCI3) δ 1.64 (bs,
1 H), 2.92-2.94 (m, 4H), 3.02-3.04 (m, 4H), 5.53 (bs, 2H)1 6.38-6.45 (m, 2H), 6.80 (t, J=8.5, 1 H).
Benzyl 4-(4-((benzyloxy)carbonyl)piperazin-1 -yl)-3-fluorophenylcarbamate (10).
Compound 10 was obtained in 78% yield (light yellow solid) using the protocol described in J. Med. Chem. 1996, 39, 673-679. 1H NMR (400 MHz, CDCI3) δ 2.98 (bs, 4H), 3.65-3.68 (m, 4H),
5.16 (s, 2H), 5.19 (s, 2H), 6.59 (bs, 1H), 6.85 (t, J=9.1 , 1 H), 6.94-6.97 (m, 1 H), 7.27-7.41 (m,
11H).
Benzyl 4-(2-fluoro-4-((R)-5-(hydroxymethyl)-2-oxo-oxazolidin-3-yl)phenyl) piperazine-1-carboxylate (11). Compound 11 was obtained in 66% yield (off-white solid) using the protocol described in J. Med. Chem. 1996, 39, 673-679. 1H NMR (400 MHz, CDCI3) δ 3.01
(bs, 4H), 3.66-3.69 (m, 4H), 3.74-3.79 (m, 1H)1 3.92-4.03 (m, 3H), 4.71-4.77 (m, 1H), 5.16 (s,
2H), 6.91 (t, J=9.1 , 1 H), 7.11-7.14 (m, 1H), 7.91-7.38 (m, 5H), 7.46 (dd, J=14.2, 2.5, 1 H).
Benzyl 4-(2-fluoro-4-((/?)-5-(methanesulfonyloxymethyl)-2-oxo-oxazolidin-3- yl)phenyl) piperazine-1-carboxylate (12). Compound 12 was obtained in quantitative yield (off- white foam) using the protocol described in J. Med. Chem. 1996, 39, 673-679. 1H NMR (400 MHz, CDCI3) δ 3.02 (bs, 4H), 3.10 (s, 3H), 3.67-3.69 (m, 4H), 3.92 (dd, J=9.1 , 6.1 , 1 H), 4.12 (t, J=QA, 1H), 4.44 (dd, J=11.7, 3.8, 1H), 4.49 (dd, J=11.7, 3.8, 1H), 4.88-4.94 (m, 1H), 5.16 (s, 2H), 6.93 (t, J=9.1 , 1 H), 7.08-7.12 (m, 1 H), 7.30-7.38 (m, 5H), 7.44 (dd, J=14.0, 2.6, 1 H).
Benzyl 4-(4-((S)-5-(aminomethyl)-2-oxo-oxazolidin-3-y!)-2-fluorophenyl) piperazine- 1-carboxylate (13). Compound 13 was obtained in 70% yield from 12 (4.4 g, 8:67 mmol), following the same procedure as for compound 6. After work-up, crude 13 was purified by flash chromatography using a gradient of 0-2-5-10% MeOH / CHCI3 as eluent. 1H NMR (400 MHz,
CDCI3) δ 1.33 (bs, 2H), 2.94-3.03 (m, 5H), 3.11 (dd, J=13.7, 4.1 , 1 H), 3.66-3.69 (m, 4H)1 3.82
(dd, J=8.6, 6.7, 1 H), 4.00 (t, J=8.7, 1 H)14.63-4.69 (m, 1 H), 5.16 (s, 2H), 6.91 (t, J=9.1 , 1 H)17.12- 7.15 (m, 1 H)1 7.30-7.38 (m, 5H)1 7.47 (dd, J=14.3, 2.6, 1 H).
Benzyl 4-(4-((S)-5-(acetylaminomethyl)-2-oxo-oxazolidin-3-yl)-2-fluorophenyl) piperazine-1-carboxylate (14). Compound 14 was obtained in 90% yield from 13 (5.3 g, 12.4 mmol), following the same procedure as for compound 7. After work-up, the compound was used without any further purification. 1H NMR (400 MHz, CDCI3) δ 2.02 (s, 3H), 3.01 (bs, 4H), 3.57-3.77 (m, 7H)14.01 (t, J=9.0, 1 H)14.73-4.79 (m, 1 H)1 5.16 (s, 2H)16.05 (t, J=6.2, 1H)16.91 (t, J=9.2, 1H), 7.05-7.08(m, 1 H)1 7.32-7.38 (m, 5H)1 7.44 (dd, J=14.2, 2.62, 1 H).
Λ/-[((S)-3-(3-fluoro-4-(piperazin-1-yl)phenyl]-2-oxo-oxazolidin-5-yl)methyl)acetamide (15). To a solution of 14 (748 mg, 1.59 mmol) in abs. ethanol (40 ml.) was added cyclohexene (1 ml.) and 10% Pd / C (400 mg). The mixture was refluxed for 2 h, when TLC indicated complete reaction. The reaction mixture was filtered through celite and concentrated to give 15 as an off-white solid (520 mg, 97%). The product was essentially pure, but could be purified by chromatography (90:10:1.5 CH2CI2:MeOH:conc. NH4OH). 1H NMR (400 MHz, CDCI3) 52.01 (s, 3H), 3.02 (d, J=Al, 8H), 3.57-3.76 (m, 3H), 4.01 (t, J=9.0, 1H), 4.73-4.79 (m, 1H), 6.29 (m, 1H)1 6.92 (t, J=9.1 , 1 H), 7.04-7.07(m, 1 H), 7.39-7.43 (m, 1 H).
Λ/-(((S)-3-(4-(4-(2-(benzyloxy)acetyl)piperazin-1-yl)-3-fluorophenyl)-2-oxooxazolidin- 5-yl)methyl)acetamide (16). To a solution of 15 (537 mg, 1.60 mmol) and triethylamine (0.22 mL, 3.53 mmol) in CH2CI2 (35 mL) at 0 0C was added benzyloxyacetyl chloride (0.30 ml_, 1.92 mmol). The mixture was stirred at 0 0C for 1 h, then 15 min at room temperature when TLC indicated complete reaction. The reaction mixture was washed with water (2 x 30 mL), and saturated sodium bicarbonate (2 x 30 mL), and dried over MgSO4. After chromatography (gradient elution 5-10% MeOH / CH2CI2) the product was obtained as a white foam (709 mg, 91%). 1H NMR (400 MHz, CDCI3) δ 2.02 (s, 3H), 2.98-3.14 (m, 4H), 3.56-3.86 (m, 7H), 4.02 (t, J=9.0, 1 H), 4.22 (S, 2H), 4.62 (s, 2H), 4.73-4.80 (m, 1H)1 6.02 (t, J=5.9,1 H), 6.96-7.10 (m, 2H), 7.28-7.40 (m, 5H), 7.45-7.53 (m, 1 H).
Λ/-(((S)-3-(3-fluoro-4-(4-(2-hydroxyacetyl)piperazin-1-yl)phenyl)-2-oxooxazoiidin-5- yl)methyl)acetamide (17, eperezolid). To a solution of 16 (709 mg, 1.46 mmol) in abs. ethanol (40 mL) was added cyclohexene (1 mL) and 10% Pd / C (250 mg). The mixture was refluxed for 15 h, when TLC indicated complete reaction. The reaction mixture was filtered through Celite™ and concentrated to give 17 (470 mg, 82% yield). The product was essentially pure, but could be purified by chromatography. 1H NMR (400 MHz, CDCI3) δ 2.02 (s, 3H), 3.06-3.10 (m, 4H), 3.45-3.50 (m, 2H), 3.58-3.77 (m, 3H), 3.85-3.87 (m, 2H), 4.02 (t, J=9.0, 1 H), 4.21 (s, 2H), 4.74- 4.80 (m, 1H), 6.09 (t, J=6.0, 1 H), 6.97 (t, J=QA , 1 H), 7.07-7.10 (m, 1 H), 7.46-7.50 (m, 1 H). LCMS : 96.1% (254 nm), 95.1% (220 nm), 94.5% (320 nm). MS : 395 (MH)+.
……………….
http://www.google.com/patents/WO1997037980A1
EXAMPLE 8 (S)-N-[[3-[3-fluoro-4- 4-(hydro3ζyacetyl)-l-piperazinyl]-phenyl]-2- oxo-5-oxazoHdinyl]methylJ-acetamide sesquihydrate (VIII) To a stirred mixture of (S)-N-[[3-[3-fluoro-4-(l-piperazinyl)phenyl]-2-oxo-5- oxazoHdinyl]methyl]acetamide hydrochloride (EXAMPLE 7, 16.2 kg, 43.5 moles), tetrahydrofuran (205 kg) and triethylamine (10.1 kg, 100 moles) is added acetoxyacetyl chloride (6.5 kg, 47.8 moles) in tetrahydrofuran (11.1 kg) over 35 minutes keeping the temperature at 22-23°. After 40 minutes, at which time TLC and HPLC analysis indicated complete formation of the acetoxyacetamide intermediate, the mixture is concentrated under reduced pressure to 30 1, diluted with methanol (100 1) and concentrated to 30 1. To the residue is added methanol (25 1) and an aqueous solution of potassium carbonate (5.6 kg in 56 1). The resulting mixture is stirred 20 hr at 22-25° at which time TLC and HPLC analysis indicates the reaction is complete. The pH is adjusted to 7-7.5 with hydrochloric acid (4 N, 14.3 1). The mixture is stirred 18 hr at 15-22° then 3 hrs at 2-5°. The soHds are collected on a filter, washed with water (68 1) and dried at 20-25° with recycled nitrogen to give the desired product. The crude product is dissolved in water (225 1) at 60-70°, clarified through a 0.6 micron filter, diluted with water rinse (55 1) and stirred 17 hrs. at 15°. The solids are collected on a filter, washed with water at 15° and dried at 45° with recycled nitrogen to a water content of 0.33%. These soHds are dissolved in a solution of ethyl acetate (143 1), methanol (65 1) and water (1.95 1) at 60-65°. The solution is cooled to 15-25° and stirred 16 hrs for crystallization. The soHds are coUected on a filter, washed with ethyl acetate (75 1) and dried with 45° nitrogen to give the desired product. The product is recrystallized two more times from water (147 1 then 133 1) at 60-70°, clarified each time through a 0.6 micron filter and rinsed with water (40 1 and 30 1). The soHds are dried on the filter at 30° with recycled nitrogen to give, after deagglomeration through a mill, the title compound as the sesquihydrate (6.45% water), TLC (siHca gel; methanol/methylene chloride, 5/95) Rf = 0.45; [α]D = -20° (c = 1.0, ethanol).

pamidronate eperezolid
|
12-8-2000
|
BICYCLIC OXAZOLIDINONES AS ANTIBACTERIAL AGENT
|
|
|
8-4-2000
|
ASSAYS FOR MODULATORS OF ELONGATION FACTOR P ACTIVITY
|
|
|
3-22-2000
|
Method of treating psoriasis, arthritis and reducing the toxicity of cancer chemotherapy
|
|
|
12-17-1999
|
MULTIVALENT MACROLIDE ANTIBIOTICS MULTIVALENT MACROLIDE ANTIBIOTICS MULTIVALENT MACROLIDE ANTIBIOTICS
|
|
8-4-2004
|
BICYCLIC HETEROCYCLIC SUBSTITUTED PHENYL OXAZOLIDINONE ANTIBACTERIALS, AND RELATED COMPOSITIONS AND METHODS
|
|
|
9-12-2003
|
Bicyclic heterocyclic substituted phenyl oxazolidinone antibacterials, and related compositions and methods
|
|
|
8-20-2003
|
Bicyclic heterocyclic substituted phenyl oxazolidinone antibacterials, and related compositions and methods
|
|
|
4-9-2003
|
Compositions and methods for treating bacterial infections
|
|
|
2-12-2003
|
Piperidinyloxy and pyrrolidinyloxy oxazolidinone antibacterials
|
|
|
2-5-2003
|
Oxazolidinone tablet formulation
|
|
|
7-3-2002
|
Bicyclic heterocyclic substituted phenyl oxazolidinone antibacterials, and related compositions and methods
|
|
|
11-30-2001
|
Treatment of urinary tract infections with antibacterial oxazolidinones
|
|
|
10-3-2001
|
N-substituted amidine and guanidine oxazolidinone antibacterials and methods of use thereof
|
|
|
6-27-2001
|
Enhancement of oxazolidinone antibacterial agents activity by using arginine derivatives
|
|
8-15-2012
|
Oxazolidinone derivatives with cyclic amidoxime or cyclic amidrazone pharmaceutical compositions thereof
|
|
|
10-20-2010
|
Oxazolidinone derivatives
|
|
|
7-31-2009
|
NOVEL OXAZOLIDINONE DERIVATIVES
|
|
|
6-20-2008
|
PREPARATION AND UTILITY OF SUBSTITUTED OXZOLIDINONES
|
|
|
9-19-2007
|
Antibiotic conjugates
|
|
|
3-31-2006
|
Antibiotic conjugates
|
|
|
10-5-2005
|
Pyridoarylphenly oxazolidinone antibacterials, and related compositions and methods
|
|
|
4-8-2005
|
Container for linezolid intravenous solution
|
|
|
1-21-2005
|
Substituted isoxazoles and their use as antibiotics
|
|
|
9-29-2004
|
Container for linezolid intravenous solution
|
Filed under: Uncategorized Tagged: EPEREZOLID