
FDA approves first treatment for certain patients with Erdheim-Chester Disease, a rare blood cancer
The U.S. Food and Drug Administration today expanded the approval of Zelboraf (vemurafenib) to include the treatment of certain adult patients with Erdheim-Chester Disease (ECD), a rare cancer of the blood. Zelboraf is indicated to treat patients whose cancer cells have a specific genetic mutation known as BRAF V600. This is the first FDA-approved treatment for ECD. Continue reading.
//////Zelboraf, vemurafenib, fda 2017, Erdheim-Chester Disease,
 |
|
 |
|
| Clinical data | |
|---|---|
| Pronunciation | /ˌvɛməˈræfənɪb/ VEM-ə-RAF-ə-nib |
| Trade names | Zelboraf |
| Synonyms | PLX4032, RG7204, RO5185426 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a612009 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| ECHA InfoCard | 100.226.540 |
| Chemical and physical data | |
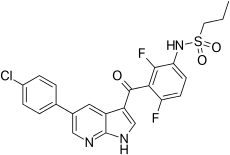
| Formula | C23H18ClF2N3O3S |
| Molar mass | 489.92 g/mol |
| 3D model (JSmol) | |
Vemurafenib (INN, marketed as Zelboraf) is a B-Raf enzyme inhibitor developed by Plexxikon (now part of Daiichi-Sankyo) and Genentech for the treatment of late-stage melanoma.[1] The name “vemurafenib” comes from V600E mutated BRAF inhibition.
Approvals
Vemurafenib received FDA approval for the treatment of late-stage melanoma on August 17, 2011,[2] making it the first drug designed using fragment-based lead discovery to gain regulatory approval.[3]
Vemurafenib later received Health Canada approval on February 15, 2012.[4]
On February 20, 2012, the European Commission approved vemurafenib as a monotherapy for the treatment of adult patients with BRAF V600E mutation positive unresectable or metastatic melanoma, the most aggressive form of skin cancer.[5]
Mechanism of action
Vemurafenib causes programmed cell death in melanoma cell lines.[6] Vemurafenib interrupts the B-Raf/MEK step on the B-Raf/MEK/ERK pathway − if the B-Raf has the common V600E mutation.
Vemurafenib only works in melanoma patients whose cancer has a V600E BRAF mutation (that is, at amino acid position number 600 on the B-Raf protein, the normal valine is replaced by glutamic acid).[7] About 60% of melanomas have this mutation. It also has efficacy against the rarer BRAF V600K mutation. Melanoma cells without these mutations are not inhibited by vemurafenib; the drug paradoxically stimulates normal BRAF and may promote tumor growth in such cases.[8][9]
Resistance
Three mechanisms of resistance to vemurafenib (covering 40% of cases) have been discovered:
- Cancer cells begin to overexpress cell surface protein PDGFRB, creating an alternative survival pathway.
- A second oncogene called NRAS mutates, reactivating the normal BRAF survival pathway.[10]
- Stromal cell secretion of hepatocyte growth factor (HGF).[11][12]
Clinical trials
In a phase I clinical study, vemurafenib (then known as PLX4032) was able to reduce numbers of cancer cells in over half of a group of 16 patients with advanced melanoma. The treated group had a median increased survival time of 6 months over the control group.[13][14][15][16]
A second phase I study, in patients with a V600E mutation in B-Raf, ~80% showed partial to complete regression. The regression lasted from 2 to 18 months.[17]
In early 2010 a Phase I trial[18] for solid tumors (including colorectal cancer), and a phase II study (for metastatic melanoma) were ongoing.[19]
A phase III trial (vs dacarbazine) in patients with previously untreated metastatic melanoma showed an improved rates of overall and progression-free survival.[20]
In June 2011, positive results were reported from the phase III BRIM3 BRAF-mutation melanoma study.[21] The BRIM3 trial reported good updated results in 2012.[22]
Further trials are planned including a trial of vemurafenib co-administered with GDC-0973 (cobimetinib), a MEK-inhibitor.[21] After good results in 2014 the combination was submitted to the EC and FDA for marketing approval.[23]
In January 2015 trial results compared vemurafenib with the combination of dabrafenib and trametinib for metastatic melanoma.[24]
Side effects
At the maximum tolerated dose (MTD) of 960 mg twice a day 31% of patients get skin lesions that may need surgical removal.[1] The BRIM-2 trial investigated 132 patients; the most common adverse events were arthralgia in 58% of patients, skin rash in 52%, and photosensitivity in 52%. In order to better manage side effects some form of dose modification was necessary in 45% of patients. The median daily dose was 1750 mg, 91% of the MTD.[25]
A trial combining vemurafenib and ipilimumab was stopped in April 2013 because of signs of liver toxicity.[26]
References
- ^ Jump up to:a b c PDB: 3OG7; Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, Spevak W, Zhang C, Zhang Y, Habets G, et al. (September 2010). “Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma”. Nature. 467 (7315): 596–599. doi:10.1038/nature09454. PMC 2948082
![Freely accessible Freely accessible]() . PMID 20823850.
. PMID 20823850. - Jump up^ “FDA Approves Zelboraf (Vemurafenib) and Companion Diagnostic for BRAF Mutation-Positive Metastatic Melanoma, a Deadly Form of Skin Cancer” (Press release). Genentech. Retrieved 2011-08-17.
- Jump up^ Bollag G, Tsai J, Zhang J, Zhang C, Ibrahim P, Nolop K, Hirth P (November 2012). “Vemurafenib: the first drug approved for BRAF-mutant cancer”. Nat Rev Drug Discov. 11 (11): 873–86. doi:10.1038/nrd3847. PMID 23060265.
- Jump up^ Notice of Decision for ZELBORAF
- Jump up^ Hofland P (February 20, 2012). “First Personalized Cancer Medicine Allows Patients with Deadly Form of Metastatic Melanoma to Live Significantly Longer”. Onco’Zine. The International Cancer Network.
- Jump up^ Sala E, Mologni L, Truffa S, Gaetano C, Bollag GE, Gambacorti-Passerini C (May 2008). “BRAF silencing by short hairpin RNA or chemical blockade by PLX4032 leads to different responses in melanoma and thyroid carcinoma cells”. Mol. Cancer Res. 6 (5): 751–9. doi:10.1158/1541-7786.MCR-07-2001. PMID 18458053.
- Jump up^ Maverakis E, Cornelius LA, Bowen GM, Phan T, Patel FB, Fitzmaurice S, He Y, Burrall B, Duong C, Kloxin AM, Sultani H, Wilken R, Martinez SR, Patel F (2015). “Metastatic melanoma – a review of current and future treatment options”. Acta Derm Venereol. 95 (5): 516–524. doi:10.2340/00015555-2035. PMID 25520039.
- Jump up^ Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, Ludlam MJ, Stokoe D, Gloor SL, Vigers G, Morales T, Aliagas I, Liu B, Sideris S, Hoeflich KP, Jaiswal BS, Seshagiri S, Koeppen H, Belvin M, Friedman LS, Malek S (February 2010). “RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth”. Nature. 464 (7287): 431–5. doi:10.1038/nature08833. PMID 20130576.
- Jump up^ Halaban R, Zhang W, Bacchiocchi A, Cheng E, Parisi F, Ariyan S, Krauthammer M, McCusker JP, Kluger Y, Sznol M (February 2010). “PLX4032, a Selective BRAF(V600E) Kinase Inhibitor, Activates the ERK Pathway and Enhances Cell Migration and Proliferation of BRAF(WT) Melanoma Cells”. Pigment Cell Melanoma Res. 23(2): 190–200. doi:10.1111/j.1755-148X.2010.00685.x. PMC 2848976
![Freely accessible Freely accessible]() . PMID 20149136.
. PMID 20149136. - Jump up^ Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, Chen Z, Lee MK, Attar N, Sazegar H, Chodon T, Nelson SF, McArthur G, Sosman JA, Ribas A, Lo RS (November 2010). “Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation”. Nature. 468 (7326): 973–977. doi:10.1038/nature09626. PMC 3143360
![Freely accessible Freely accessible]() . PMID 21107323. Lay summary – Genetic Engineering & Biotechnology News.
. PMID 21107323. Lay summary – Genetic Engineering & Biotechnology News. - Jump up^ Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, Davis A, Mongare MM, Gould J, Frederick DT, Cooper ZA, Chapman PB, Solit DB, Ribas A, Lo RS, Flaherty KT, Ogino S, Wargo JA, Golub TR (July 2012). “Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion”. Nature. 487 (7408): 500–4. doi:10.1038/nature11183. PMC 3711467
![Freely accessible Freely accessible]() . PMID 22763439.
. PMID 22763439. - Jump up^ Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, Peng J, Lin E, Wang Y, Sosman J, Ribas A, Li J, Moffat J, Sutherlin DP, Koeppen H, Merchant M, Neve R, Settleman J (July 2012). “Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors”. Nature. 487 (7408): 505–9. doi:10.1038/nature11249. PMC 3724525
![Freely accessible Freely accessible]() . PMID 22763448.
. PMID 22763448. - Jump up^ “Drug hope for advanced melanoma”. BBC News. 2009-06-02. Retrieved 2009-06-07.
- Jump up^ Harmon, Amy (2010-02-21). “A Roller Coaster Chase for a Cure”. The New York Times.
- Jump up^ Garber K (December 2009). “Melanoma drug vindicates targeted approach”. Science. 326 (5960): 1619. doi:10.1126/science.326.5960.1619. PMID 20019269.
- Jump up^ Flaherty K. “Phase I study of PLX4032: Proof of concept for V600E BRAF mutation as a therapeutic target in human cancer”. 2009 ASCO Annual Meeting Abstract, J Clin Oncol 27:15s, 2009 (suppl; abstr 9000).
- Jump up^ Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O’Dwyer PJ, Lee RJ, Grippo JF, Nolop K, Chapman PB (August 2010). “Inhibition of mutated, activated BRAF in metastatic melanoma”. N. Engl. J. Med. 363 (9): 809–19. doi:10.1056/NEJMoa1002011. PMID 20818844. Lay summary – Corante: In the Pipeline.
- Jump up^ “Safety Study of PLX4032 in Patients With Solid Tumors”. ClinicalTrials.gov.
- Jump up^ “A Study of RO5185426 in Previously Treated Patients With Metastatic Melanoma”. ClinicalTrials.gov. 2010-02-15.
- Jump up^ “Plexxikon Announces First Patient Dosed in Phase 3 Trial of PLX4032 (RG7204) for Metastatic Melanoma” (Press release). Plexxikon. 2010-01-08.
- ^ Jump up to:a b “Plexxikon and Roche Report Positive Data from Phase III BRAF Mutation Melanoma Study”. 6 June 2011.
- Jump up^ “Vemurafenib Improves Overall Survival in Patients with Metastatic Melanoma”.
- Jump up^ Cobimetinib at exelixis.com
- Jump up^ “MEK/BRAF Inhibitor Combo Reduces Death by One-Third in Melanoma”. 2015.
- Jump up^ “BRIM-2 Upholds Benefits Emerging with Vemurafenib in Melanoma”. Oncology & Biotech News. 5 (7). July 2011.
- Jump up^ “Getting close and personal”. The Economist. January 4, 2014. ISSN 0013-0613. Retrieved 2016-04-15.
Filed under: FDA 2017 Tagged: Erdheim-Chester Disease, FDA 2017, vemurafenib, Zelboraf

