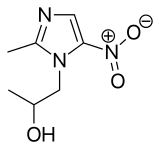
Secnidazole
- Molecular FormulaC7H11N3O3
- Average mass185.180 Da
Solosec (secnidazole) ; Symbiomix Therapeutics; For the treatment of bacterial vaginosis , Approved September 2017
Company: Symbiomix Therapeutics
Approval Status: Approved FDA September 2017
Specific Treatments: bacterial vaginosis
Therapeutic Areas Obstetrics/Gynecology (Women’s Health)
Infections and Infectious Diseases
Secnidazole is a second-generation 5-nitroimidazole antimicrobial that is structurally related to other 5-nitroimidazoles including Metronidazole and Tinidazole, but displays improved oral absorption and longer terminal elimination half-life than antimicrobial agents in this class [1]. Secnidazole is selective against many anaerobic Gram-positive and Gram-negative bacteria and protozoa. In September 2017, FDA granted approval to secnidazole under the market name Solosec as a single-dose oral treatment for bacterial vaginosis, which is a common vaginal infection in women aged 15 to 44 years. The antimicrobial therapy is only intended to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria [FDA Label].
Secnidazole (trade names Flagentyl, Sindose, Secnil) is a nitroimidazole anti-infective. Effectiveness in the treatment of dientamoebiasis has been reported.[1] It has also been tested against Atopobium vaginae.[2]
Mechanism of Action
Solosec (secnidazole) is a 5-nitroimidazole antimicrobial. 5-nitroimidazoles enter the bacterial cell as an inactive prodrug where the nitro group is reduced by bacterial enzymes to radical anions. It is believed that these radical anions interfere with bacterial DNA synthesis of susceptible isolates.
DE 2107405; FR 2079880; GB 1278757; JP 49080066

The condensation of (I) with propylene oxide (A) in ethanol at 20 C gives 1-(2-hydroxypropyl)-2-methylimidazole (III), which is acetylated with acetyl chloride in refluxing acetonitrile yielding the corresponding acetate (IV). The nitration of (IV) by means of HNO3 and P2O5 affords 1-(2-acetoxypropyl)-2-methyl-4-nitroimidazole (V), which is finally hydrolyzed with 4N HCl at 90 C
CH 513177; DE 2107423; FR 2079879; GB 1278758; NL 7101641

The reaction of (I) with chloroacetone (C) by means of K2CO3 in refluxing acetone gives (2-methylimidazol-1-yl)acetone (VI), which is nitrated with HNO3 and P2O5 affording the corresponding nitro compound (VII). Finally, this product is reduced with NaBH4 in methanol at room temperature.
| Drugs Fut 1979,4(4),280, Arzneim-Forsch Drug Res 1966,16(1),23-29 |
The nitration of (I) with HNO3 and H2SO4 gives 2-methyl-4(5)-nitroimidazole (II), which is then condensed with refluxing 1-chloroisopropanol (B) or with propylene oxide in 85% formic acid (A).
References
- Jump up^ Girginkardeşler, N.; Coşkun, S.; Cüneyt Balcioğlu, I.; Ertan, P.; Ok, U. Z. (2003). “Dientamoeba fragilis, a neglected cause of diarrhea, successfully treated with secnidazole”. Clinical Microbiology and Infection. 9 (2): 110–113. PMID 12588330. doi:10.1046/j.1469-0691.2003.00504.x.
- Jump up^ De Backer, E.; Dubreuil, L.; Brauman, M.; Acar, J.; Vaneechoutte, M. (2009). “In vitro activity of secnidazole against Atopobium vaginae, an anaerobic pathogen involved in bacterial vaginosis”. Clinical Microbiology and Infection. 16 (5): 470–472. PMID 19548924. doi:10.1111/j.1469-0691.2009.02852.x.
External links
- Gillis, J. C.; Wiseman, L. R. (1996). “Secnidazole. A review of its antimicrobial activity, pharmacokinetic properties and therapeutic use in the management of protozoal infections and bacterial vaginosis”. Drugs. 51 (4): 621–38. PMID 8706597. doi:10.2165/00003495-199651040-00007.
 |
|
| Clinical data | |
|---|---|
| Synonyms | PM 185184, RP 14539 |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration |
Oral |
| ATC code | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.020.123 |
| Chemical and physical data | |
| Formula | C7H11N3O3 |
| Molar mass | 185.180 g/mol |
| 3D model (JSmol) | |
////////////Secnidazole, секнидазол , سيكنيدازول , 塞克硝唑 , FDA 2017, RP-14539, PM-185184, Flagentyl
CC1=NC=C(N1CC(C)O)[N+](=O)[O-]
Filed under: FDA 2017 Tagged: FDA 2017, Flagentyl, секнидазол, PM-185184, RP-14539, Secnidazole, 塞克硝唑, سيكنيدازول


