
Licorice or Mulethi is a medicinal herb which is used in various Ayurvedic medicines. Its underground stems and roots are used for medicinal purpose. It has antioxidant, antimicrobial, anti-inflammatory and hepatoprotective properties.
Mulethi is useful in cough, sore throat, bronchitis, sexual weakness, skin problems, jaundice, hoarseness, vata dosha, ulcers etc. It has demulcent and expectorant properties.
read…………MY OLD ARTICLE

Liquorice, or licorice, (/ˈlɪk(ə)rɪʃ/ lik-(ə-)rish or /ˈlɪk(ə)rɪs/ lik-(ə-)ris)[2] is the root of Glycyrrhiza glabra from which a sweet flavour can be extracted. The liquorice plant is a legume native to southern Europe, India, and parts of Asia. It is not botanically related to anise, star anise, or fennel, which are sources of similar flavouring compounds. The word liquorice / licorice is derived (via the Old French licoresse) from the Greek γλυκύρριζα (glukurrhiza), meaning “sweet root”,[3] from γλυκύς (glukus), “sweet”[4] + ῥίζα (rhiza), “root”,[5][6] the name provided by Dioscorides.[7] It has been traditionally known and used as medicine in Ayurveda for rejuvenation.[8] It is called asadhimadhuram (அதிமதுரம்) in Tamil, irattimadhuram in Malayalam, yastimadhu (यस्टिमधु) in Sanskrit, mulethi (मुलेठी) in Hindi, andjethimadh (જેઠીમધ) in Gujarati language.[9]
Licorice (Glycyrrhiza glabra), locally known as mulethi, has been revered for centuries as a medicinal herb in Ayurveda. Besides possessing numerous medicinal properties, it is also a popular flavoring herb as it is 50 times sweeter than sugar, due to the presence of a compound called glycyrrhizin.
Through research, the anti-oxidant, anti-inflammatory, anti-microbial, analgesic (pain-relieving) and expectorant properties of this is sweet, moist herb have been established worldwide. It is also diuretic, rejuvenating and mildly laxative in nature. These properties have helped Licorice find a place in both Eastern and Western medicine for treating an array of ailments, ranging from cold and cough to arthritis, respiratory, digestive and liver problems.

The Sanskrit name for licorice is Yashtimadhu, which literally means “sweet root”. It is sweet, cool and heavy to digest. The Rasa (taste) of this herb is madhura (sweet), which makes it beneficial for vata and pitta doshas, while it’s Virya (action) is sheetal (cooling), which generally increases kapha when consumed in large doses over long term.
The medicinal property of mulethi is mainly because of the presence of powerful phytochemicals namely flavonoids, chalcones, saponins and xenoestrogens. Glycyrrhizin (salts of glycyrrhizic acid) is a popular saponin found in roots of mulethi that is responsible for the characteristic sweet taste (50 times more sweet than sugar) flavor. Liquiritin, licoflavonol, liquiritigenin, etc are the common chalcones that provide the distinct yellowish color to mulethi; while, the aroma of its root is mainly because of anethole. Here are the ten health benefits of mulethi:

Information
Latin name: Glycyrrhiza glabra
Sanskrit: Madhuyashti
Hindi: Mulhatti, Jethimadh, Mithilakdi
English: Sweetwood, Liquorice, Licorice
Bengali: Jashtimadhu
Gujrati: Jethi Madh
Marathi: Jeshtamadhu
Kannada: Jeshthamadhu
Malayalam: Itarttimadhuram, Erattimadhuram
Tamil: Atimadhuram
Telugu: Atimadhuramu

Anti-microbial activity – Roots of mulethi are very effective in protecting against virus, bacteria and fungi due to the presence of Glycyrrhizin that blocks the microbial growth. The root extract possesses the power to control malaria (as per preliminary research), influenza and also helps in the treatment of herpes resulting in virus suppression and severity of sores.
Anti-inflammatory activity – Liquorice has powerful anti-inflammatory and anti-allergic activity and can be used to treat chronic inflammation like rheumatic problems & arthritis, skin diseases and autoimmune diseases. It is also used for preventing any inflammatory conditions related to eye and also to treat conjunctivitis with the help of glycyrrhizin activity that counteracts negative effects caused by cortisol.
Improves immunity – Root extracts of mulethi aids in increasing the production of lymphocytes and macrophage thereby improving your defense mechanism & preventing microbial attack. It also helps in minimizing immune related allergic reactions and autoimmune complications.

Memory improvement – Roots of licorice exert supportive effect on the adrenal gland and thus indirectly aid in stimulating the brain. It not only decreases the effects of amnesia & improves learning but its antioxidant property (mulethi contains flavonoids) renders a shielding effect on the brain cells.
Anti-ulcer activity – The potent antioxidant and anti-inflamatory properties of licorice makes it the best natural medicinal aid to treat ulcers of stomach, intestine and mouth. The compound carbenoxolone synthesized from glycyrrhizin plays key role in healing mouth and gastric ulcers along with reducing gastric secretions and promoting development of intestinal mucus lining.
Liver protection – Licorice is one of the most common traditional remedy used to treat jaundice. Its antioxidant property is the key for preventing your liver from the action of free radicals and toxic materials. This herb is also reported to exhibit protection against diclofenac induced toxicity and also, in inhibiting damage of liver.

Digestive aid – Roots of licorice are also used to deal with stomach and digestion problems with the help of glycyrrhizin and its compound, carbenoxolone. It is one of the ancient home remedies for relieving constipation, acidity, heartburn, stomach discomfort, inflammation of digestive system and gastro esophageal acid reflux. As a mild laxative, it plays an effective role in bowel movements and also for treatment of allergic cough in addition to maintaining normal pH levels.
Hormonal regulation – The phytoestrogenic compounds present in mulethi roots exert valuable action against women hormonal imbalance problems, menopause symptoms like hot flashes & exhaustion, mood swings, etc. It is also found to help in cortisol production and relieving premenstrual issues like nausea and menstrual cramps. Licorice powder acts as the traditional medicine for nursing mothers to regulate body hormones and aid in milk secretion.
Heart healthy effects – Research studies have proved that licorice roots help in controlling cholesterol levels by increasing the body’s flow of bile and also reducing high blood cholesterol levels. The anti-oxidant property of licorice acts in increasing the blood capillary health, reducing inflammation, prevents blood vessel damage and block development of arterial plaque.
Other effects – Licorice roots work wonders in treatment of depression, diabetes and respiratory tract infection like sore throat (hoarseness of voice), cold and cough, etc in addition to rendering effective skin benefits, oral hygiene and weight loss. It is found to act as a cancer cure remedy, a potent aphrodisiac and a powerful analgesic agent.
Description
It is a herbaceous perennial, growing to 1 m in height, with pinnate leaves about 7–15 cm (3–6 in) long, with 9–17 leaflets. The flowers are 0.8–1.2 cm (1/3 to 1/2 in) long, purple to pale whitish blue, produced in a loose inflorescence. The fruit is an oblong pod, 2–3 cm (1 in) long, containing several seeds.[10] The roots are stoloniferous.[11]
Chemistry
The scent of liquorice root comes from a complex and variable combination of compounds, of which anethole is up to 3% of total volatiles. Much of the sweetness in liquorice comes from glycyrrhizin, which has a sweet taste, 30–50 times the sweetness of sugar. The sweetness is very different from sugar, being less instant, tart, and lasting longer.
The isoflavene glabrene and the isoflavane glabridin, found in the roots of liquorice, are phytoestrogens.[12][13]
Cultivation and uses
Liquorice, which grows best in well-drained soils in deep valleys with full sun, is harvested in the autumn two to three years after planting.[10] Countries producing liquorice include Iran, Afghanistan, the People’s Republic of China, Pakistan, Iraq, Azerbaijan, Uzbekistan, Turkmenistan, and Turkey.[14]
The world’s leading manufacturer of liquorice products is M&F Worldwide, which manufactures more than 70% of the worldwide liquorice flavours sold to end users.[15]
Safe dosage
Licorice is available in various forms – root, powder and extracts. Licorice root can be chewed directly while licorice tea (prepared by boiling licorice root in water) is also extremely beneficial as a home remedy.
Daily intake of 5-6 grams of licorice powder is considered safe while 250-500 mg of concentrated extracts can be taken thrice a day. Unsupervised use in high doses is not recommended for long term. People with hypertension or heart disease, pregnant women and breastfeeding mothers should avoid using licorice without prior consulation with an Ayurveda doctor.
 plant
plant

Medicine
The compound glycyrrhizin (or glycyrrhizic acid), found in liquorice, has been proposed as being useful for liver protection in tuberculosis therapy, but evidence does not support this use, which may in fact be harmful.[24] Glycyrrhizin has also demonstrated antiviral, antimicrobial, anti-inflammatory, hepatoprotective, and blood pressure-increasing effects in vitro and in vivo, as is supported by the finding that intravenous glycyrrhizin (as if it is given orally very little of the original drug makes it into circulation) slows the progression of viral and autoimmune hepatitis.[25][26] Liquorice has also demonstrated promising activity in one clinical trial, when applied topically, against atopic dermatitis.[27] Additionally, liquorice has also proven itself effective in treating hyperlipidaemia (a high amount of fats in the blood).[28] Liquorice has also demonstrated efficacy in treating inflammation-induced skin hyperpigmentation.[29][30] Liquorice may also be useful in preventing neurodegenerative disorders and dental caries.[31][32][33]
The antiulcer, laxative, antidiabetic, anti-inflammatory, immunomodulatory, antitumour and expectorant properties of liquorice have been investigated.[34]
Folk medicine
In traditional Chinese medicine, liquorice (मुलेठी, 甘草, شیرین بیان) is believed to “harmonize” the ingredients in a formula and to carry the formula to the 12 “regular meridians”.[35]
References
- “Glycyrrhiza glabra information from NPGS/GRIN”. http://www.ars-grin.gov. Retrieved 6 March 2008.
- licorice. Merriam-Webster’s Medical Dictionary, © 2007 Merriam-Webster, Inc.
- γλυκύρριζα, Henry George Liddell, Robert Scott, A Greek-English Lexicon, on Perseus
- γλυκύς, Henry George Liddell, Robert Scott, A Greek-English Lexicon, on Perseus
- Jump up^ ῥίζα, Henry George Liddell, Robert Scott, A Greek-English Lexicon, on Perseus<
- Jump up^ liquorice, on Oxford Dictionaries
- Jump up^ google books Maud Grieve, Manya Marshall – A modern herbal: the medicinal, culinary, cosmetic and economic properties, cultivation and folk-lore of herbs, grasses, fungi, shrubs, & trees with all their modern scientific uses, Volume 2 Dover Publications, 1982 & Pharmacist’s Guide to Medicinal Herbs Arthur M. Presser Smart Publications, 1 Apr 2001 2012-05-19
- Jump up^ Balakrishna, Acharya (2006). Ayurveda: Its Principles & Philosophies. New Delhi, India: Divya prakashan. p. 206. ISBN 8189235567.
- Jump up^ “Top 10 health benefits of Mulethi or Liquorice”.
- ^ Jump up to:a b Huxley, A., ed. (1992). New RHS Dictionary of Gardening. ISBN 0-333-47494-5
- Jump up^ Brown, D., ed. (1995). “The RHS encyclopedia of herbs and their uses”. ISBN 1-4053-0059-0
- Jump up^ Somjen, D.; Katzburg, S.; Vaya, J.; Kaye, A. M.; Hendel, D.; Posner, G. H.; Tamir, S. (2004). “Estrogenic activity of glabridin and glabrene from licorice roots on human osteoblasts and prepubertal rat skeletal tissues”. The Journal of Steroid Biochemistry and Molecular Biology 91 (4–5): 241–246. doi:10.1016/j.jsbmb.2004.04.008. PMID 15336701.
- Jump up^ Tamir, S.; Eizenberg, M.; Somjen, D.; Izrael, S.; Vaya, J. (2001). “Estrogen-like activity of glabrene and other constituents isolated from licorice root”. The Journal of steroid biochemistry and molecular biology 78 (3): 291–298. doi:10.1016/S0960-0760(01)00093-0. PMID 11595510.
- ^ Jump up to:a b c M & F Worldwide Corp., Annual Report on Form 10-K for the Year Ended December 31, 2010.
- Jump up^ M & F Worldwide Corp., Annual Report on Form 10-K for the Year Ended December 31, 2001.
- Jump up^ Erik Assadourian, Cigarette Production Drops, Vital Signs 2005, at 70.
- Jump up^ M & F Worldwide Corp., Annual Report on Form 10-K for the Year Ended December 31, 2005.
- ^ Jump up to:a b c Marvin K. Cook, The Use of Licorice and Other Flavoring Material in Tobacco (Apr. 10, 1975).
- Jump up^ Boeken v. Phillip Morris Inc., 127 Cal. App. 4th 1640, 1673, 26 Cal. Rptr. 3d 638, 664 (2005).
- Jump up^ [1] the online Dutch food composition database]
- Jump up^ “Right good food from the Ridings”. AboutFood.com. 25 October 2007.
- Jump up^ “Where Liquorice Roots Go Deep”. Northern Echo. Retrieved 9 December 2008.
- Jump up^ http://science.howstuffworks.com/life/botany/licorice-info.htm
- Jump up^ Liu Q, Garner P, Wang Y, Huang B, Smith H (2008). “Drugs and herbs given to prevent hepatotoxicity of tuberculosis therapy: systematic review of ingredients and evaluation studies”.BMC Public Health (Systematic review) 8: 365. doi:10.1186/1471-2458-8-365. PMC 2576232. PMID 18939987.
- Jump up^ Chien, CF; Wu, YT; Tsai, TH (January 2011). “Biological analysis of herbal medicines used for the treatment of liver diseases.”. Biomedical Chromatography 25 (1-2): 21–38.doi:10.1002/bmc.1568. PMID 21204110.
- Jump up^ Yasui, S; Fujiwara, K; Tawada, A; Fukuda, Y; Nakano, M; Yokosuka, O (December 2011). “Efficacy of intravenous glycyrrhizin in the early stage of acute onset autoimmune hepatitis.”.Digestive Diseases and Sciences 56 (12): 3638–47. doi:10.1007/s10620-011-1789-5. PMID 21681505.
- Jump up^ Reuter, J; Merfort, I; Schempp, CM (2010). “Botanicals in dermatology: an evidence-based review.”. American Journal of Clinical Dermatology 11 (4): 247–67. doi:10.2165/11533220-000000000-00000. PMID 20509719.
- Jump up^ Hasani-Ranjbar, S; Nayebi, N; Moradi, L; Mehri, A; Larijani, B; Abdollahi, M (2010). “The efficacy and safety of herbal medicines used in the treatment of hyperlipidemia; a systematic review.”. Current pharmaceutical design 16 (26): 2935–47. doi:10.2174/138161210793176464. PMID 20858178.
- Jump up^ Callender, VD; St Surin-Lord, S; Davis, EC; Maclin, M (April 2011). “Postinflammatory hyperpigmentation: etiologic and therapeutic considerations.”. American Journal of Clinical Dermatology12 (2): 87–99. doi:10.2165/11536930-000000000-00000. PMID 21348540.
- Jump up^ Leyden, JJ; Shergill, B; Micali, G; Downie, J; Wallo, W (October 2011). “Natural options for the management of hyperpigmentation.”. Journal of the European Academy of Dermatology and Venereology 25 (10): 1140–5. doi:10.1111/j.1468-3083.2011.04130.x. PMID 21623927.
- Jump up^ Kannappan, R; Gupta, SC; Kim, JH; Reuter, S; Aggarwal, BB (October 2011). “Neuroprotection by spice-derived nutraceuticals: you are what you eat!” (PDF). Molecular Neurobiology 44(2): 142–59. doi:10.1007/s12035-011-8168-2. PMC 3183139. PMID 21360003.
- Jump up^ Gazzani, G; Daglia, M; Papetti, A (April 2012). “Food components with anticaries activity.”. Current Opinion in Biotechnology 23 (2): 153–9. doi:10.1016/j.copbio.2011.09.003.PMID 22030309.
- Jump up^ Messier, C; Epifano, F; Genovese, S; Grenier, D (January 2012). “Licorice and its potential beneficial effects in common oro-dental diseases.”. Oral Diseases 18 (1): 32–9.doi:10.1111/j.1601-0825.2011.01842.x. PMID 21851508.
- Jump up^ Shibata, S (October 2000). “A drug over the millennia: pharmacognosy, chemistry, and pharmacology of licorice.”. Yakugaku Zasshi 120 (10): 849–62. PMID 11082698.
- Jump up^ Bensky, Dan; et al. (2004). Chinese Herbal Medicine: Materia Medica, Third Edition. Eastland Press. ISBN 0-939616-42-4.
- Jump up^ Olukoga, A; Donaldson, D (June 2000). “Liquorice and its health implications.”. The Journal of the Royal Society for the Promotion of Health 120 (2): 83–9.doi:10.1177/146642400012000203. PMID 10944880.
- Jump up^ Armanini, D; Fiore, C; Mattarello, MJ; Bielenberg, J; Palermo, M (September 2002). “History of the endocrine effects of licorice.”. Experimental and Clinical Endocrinology & diabetes 110 (6): 257–61. doi:10.1055/s-2002-34587. PMID 12373628.
- Jump up^ Omar, Hesham R; Komarova,, Irina; El-Ghonemi,, Mohamed; Ahmed, Fathy; Rashad, Rania; Abdelmalak, Hany D; Yerramadha, Muralidhar Reddy; Ali, Yaseen; Camporesi, Enrico M. “How much is too much? in Licorice abuse: time to send a warning message from Therapeutic Advances in Endocrinology and Metabolism”. http://www.ncbi.nlm.nih.gov. SAGE Publications. Retrieved 13 January 2015.
38 Toxicology Center[2]
External links
 Glycyrrhiza glabra (licorice), Kew plant profile Glycyrrhiza glabra (licorice), Kew plant profile |
- Licorice, Medline, National Institutes of Health
- What’s That Stuff?: Licorice, Chemical & Engineering News
Filed under: AYURVEDA Tagged: Anthony crasto, AYURVEDA, licorice, liquorice, mulethi



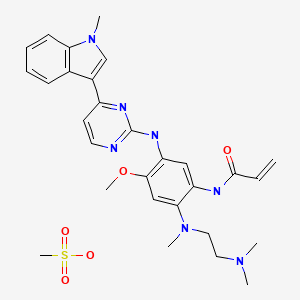





 RAMELTEON
RAMELTEON














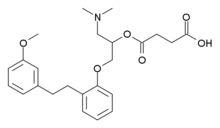



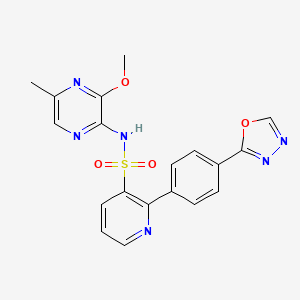
 LESINURAD
LESINURAD

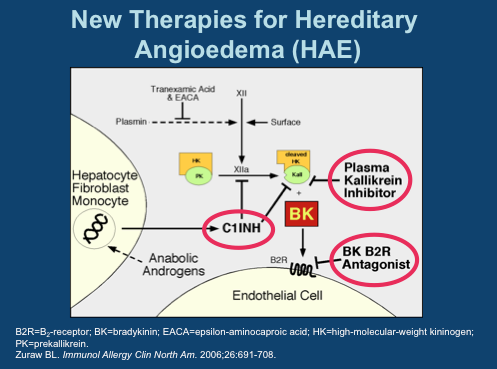
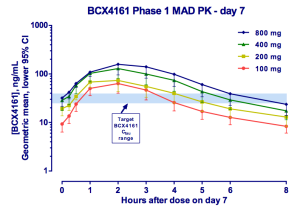
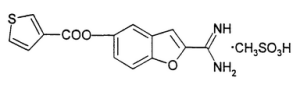
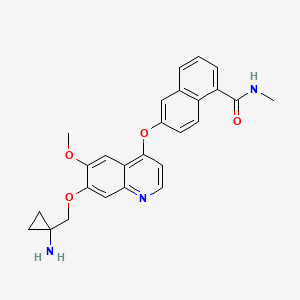
%20Structure.gif)















![6-[7-[(1-aminocyclopropyl)methoxy]-6-methoxyquinolin-4-yl]oxy-N-methylnaphthalene-1-carboxamide NMR spectra analysis, Chemical CAS NO. 1058137-23-7 NMR spectral analysis, 6-[7-[(1-aminocyclopropyl)methoxy]-6-methoxyquinolin-4-yl]oxy-N-methylnaphthalene-1-carboxamide H-NMR spectrum](http://pic11.molbase.net/nmr/nmr_image/2014-09-06/001/567/717/1058137-23-7-1h.png)
![6-[7-[(1-aminocyclopropyl)methoxy]-6-methoxyquinolin-4-yl]oxy-N-methylnaphthalene-1-carboxamide NMR spectra analysis, Chemical CAS NO. 1058137-23-7 NMR spectral analysis, 6-[7-[(1-aminocyclopropyl)methoxy]-6-methoxyquinolin-4-yl]oxy-N-methylnaphthalene-1-carboxamide C-NMR spectrum](http://pic11.molbase.net/nmr/nmr_image/2014-09-06/001/567/717/1058137-23-7-13c.png)


.png)

.jpg)























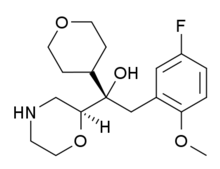




![(1R)-2-(5-fluoro-2-methoxyphenyl)-1-[(2S)-morpholin-2-yl]-1-(oxan-4-yl)ethanol NMR spectra analysis, Chemical CAS NO. 1194508-25-2 NMR spectral analysis, (1R)-2-(5-fluoro-2-methoxyphenyl)-1-[(2S)-morpholin-2-yl]-1-(oxan-4-yl)ethanol H-NMR spectrum](http://pic11.molbase.net/nmr/nmr_image/2014-09-06/001/570/465/1194508-25-2-1h.png)
![(1R)-2-(5-fluoro-2-methoxyphenyl)-1-[(2S)-morpholin-2-yl]-1-(oxan-4-yl)ethanol NMR spectra analysis, Chemical CAS NO. 1194508-25-2 NMR spectral analysis, (1R)-2-(5-fluoro-2-methoxyphenyl)-1-[(2S)-morpholin-2-yl]-1-(oxan-4-yl)ethanol C-NMR spectrum](http://pic11.molbase.net/nmr/nmr_image/2014-09-06/001/570/465/1194508-25-2-13c.png)



 Pfizer’s NYC headquarters
Pfizer’s NYC headquarters
