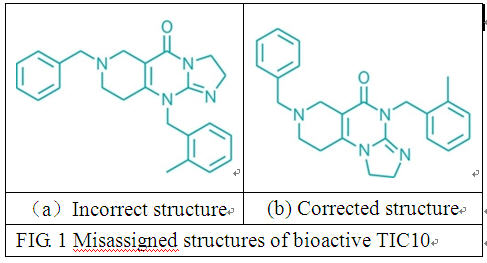
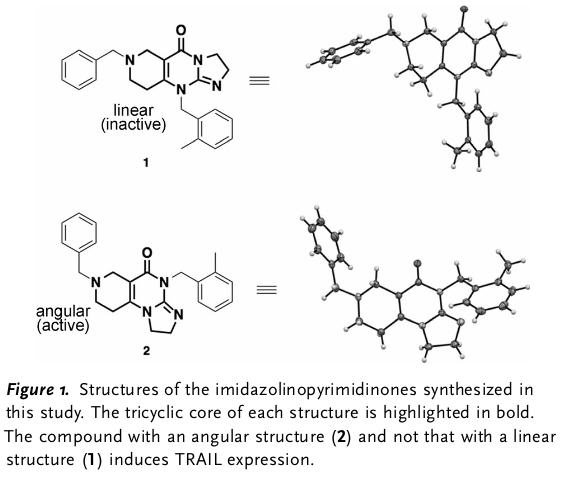
heparan sulfate mimetic derived from unfractionated heparin with a molecular weight between 5500 and 6200 Da
Necuparanib
M-402
M-ONC-402
MONC 402
http://www.pharmaceutical-technology.com/news/newsmomenta-pharma-receives-fda-orphan-drug-designation-pancreatic-cancer-drug-4287892
Momenta Pharmaceuticals has received orphan drug designation from the US Food and Drug Administration (FDA) for its necuparanib, a heparan sulfate mimetic indicated for treatment of pancreatic cancer.
Momenta Pharmaceuticals chief medical officer Jim Roach said there is a great need for new medications for patients suffering from pancreatic cancer.
“We are encouraged by the progress of the programme to date, and in the next several months, we anticipate completing Part A of our ongoing Phase I/II study of necuparanib in combination with Abraxane and gemcitabine,” Roach said.
“In the next several months, we anticipate completing Part A of our ongoing Phase I/II study of necuparanib in combination with Abraxane and gemcitabine.”
“We look forward to sharing the results from Part A and advancing the product into the Phase II part of the study in the second half of 2014.”
Necuparanib has recently been adopted as the unique non-proprietary name for M402 by The United States Adopted Names.
The drug is derived from unfractionated heparin. It has been engineered to have significantly reduced anticoagulant activity while preserving the relevant antitumor properties of heparin.
Part A dose escalation component of the Phase I/II trial, which is evaluating necuparanib in combination with Abraxane (nab-paclitaxel) and gemcitabine in advanced metastatic pancreatic cancer patients, is expected to be completed in the next several months.
The company is expected to report the clinical data from Part A in the second half this year. The company also plans to begin Part B of the study by the year-end.
Part B will be a randomised, controlled, proof-of-concept study to assess the antitumor activity of necuparanib in combination with Abraxane plus gemcitabine, versus Abraxane plus gemcitabine alone.
Heparin, a highly sulfated heparin-like glycosaniinoglycan (HLGAG) produced by mast cells and isolated from natural sources, is a widely used clinical anticoagulant. However, the effects of natural, or unfractionated, heparin can be difficult to predict and patients must be monitored closely to prevent over- or under-anticoagulation. Low molecular weight heparins (LMWHs) obtained by various methods of fractionation or depolymerization of polymeric heparin have more predictable pharmacological action as anticoagulants, reduced side effects, sustained antithrombotic activity, and better bioavailability than unfractionated heparin (UFH). Several LMWHs are approved for outpatient treatment of thrombotic conditions.
There is increasing interest in the potential role of antithrombotic agents in the management of cancer patients. Results from several recent clinical trials have suggested a survival advantage for certain types of cancer patients treated with LMWHs (reviewed in Lemoine, 2005, Journal of Clinical Oncology, 23: 2119-20).
http://www.google.fm/patents/EP2207811A1?cl=en
The invention is based, in part, on the development of polysaccharide preparations, e.g., preparations of polysaccharides derived from heparin, that lack substantial anticoagulant activity (e.g., preparations of polysaccharides that have substantially no anticoagulant activity) but retain activity in other non-coagulation mediated biological processes, and methods to produce them. These compounds can have one or more of the following features: 1) an anti-Xa activity and an anti-IIa activity each less than 50 IU/mg, and 2) anti-metastatic, anti-angiogenic, anti-fibrotic and/or anti-inflammatory activity. The polysaccharides disclosed herein can also have structural characteristics that distinguish them from other polysaccharides, (e.g., from commercially available heparins). For example, a polysaccharide preparation provided herein can have one or more of the following characteristics: the preparation has less than 50% glycol split uronic acid residues; the preparation has no more than 3 glycol split uronic acid residues (UG) per polysaccharide chain; the preparation has greater than 40% U2SHNS>6S disaccharide residues; degree of desulfation of the preparation is less than 40%; one or more polysaccharide chains in the preparation have a 4,5-unsaturation of a non-reducing end uronic acid residue; one or more polysaccharide chains in the preparation have a 2,5-anhydromannitol residue at the reducing end; and the weight average molecular weight of the preparation is between 3,500 and 7,000 Da. This disclosure includes preparations having one or more of these properties and characteristics as well as methods of making and using such preparations. The disclosure also features methods of using such preparations.
Accordingly, in a first aspect, the invention features a polysaccharide preparation (e.g., a heparin-derived preparation) having the following characteristics: (a) a weight average chain molecular weight between 3,500 and 7,000 Da; (b) an anti-Xa activity and an anti-IIa activity each less than 50 IU/mg (e.g., an anti-Xa activity less than about 40 IU/mg, 30 IU/mg, 20 IU/mg, 15 IU/mg, or 10 IU/mg and an anti-IIa activity less than about 40 IU/mg, 30 IU/mg, 20 IU/mg, 10 IU/mg, 5 IU/mg, 4 IU/mg, or 3 IU/mg); and (c) less than 50% glycol split uronic acid residues (e.g., less than 40%, 30%, 25%, or 20% glycol split uronic acid residues) in the preparation. In some embodiments, the preparation contains between 5% and 50% glycol split uronic acid residues (e.g., between 5% and 40%, 5% and 30%, 10% and 50%, 10% and 40%, or 10% and 30% glycol split uronic acid residues).
In a second aspect, the invention features a polysaccharide preparation (e.g., a heparin- derived preparation) having the following characteristics: (a) a weight average chain molecular weight between 3,500 and 7,000 Da; (b) an anti-Xa activity and an anti-IIa activity each less than 50 IU/mg (e.g., an anti-Xa activity less than about 40 IU/mg, 30 IU/mg, 20 IU/mg, 15 IU/mg, or 10 IU/mg and an anti-IIa activity less than about 40 IU/mg, 30 IU/mg, 20 IU/mg, 10 IU/mg, 5 IU/mg, 4 IU/mg, or 3 IU/mg); and (c) the polysaccharide chains of the preparation have no more than 3 glycol split uronic acid residues (UQ) per polysaccharide chain (e.g., each polysaccharide chain has no more than 2 or no more than 1 glycol split uronic acid residue (UQ) per polysaccharide chain).
In a third aspect, the invention features a polysaccharide preparation (e.g., a heparin- derived preparation) having the following characteristics: (a) a weight average chain molecular weight between 3,500 and 7,000 Da; (b) an anti-Xa activity and an anti-IIa activity each less than 50 IU/mg (e.g., an anti-Xa activity less than about 40 IU/mg, 30 IU/mg, 20 IU/mg, 15 IU/mg, or 10 IU/mg and an anti-IIa activity less than about 40 IU/mg, 30 IU/mg, 20 IU/mg, 10 IU/mg, 5 IU/mg, 4 IU/mg, or 3 IU/mg); and (c) polysaccharide chains of the preparation have on average no more than 3 glycol split uronic acid residues (Uo) per polysaccharide chain (e.g., on average no more than 2.5, no more than 2, no more than 1.5, or no more than 1 glycol split uronic acid residues (UG) per polysaccharide chain.
In a fourth aspect, the invention features a polysaccharide preparation (e.g., a heparin- derived preparation) having the following characteristics: (a) a weight average chain molecular weight between 3,500 and 7,000 Da; (b) an anti-Xa activity and an anti-IIa activity each less than 50 IU/mg (e.g., an anti-Xa activity less than about 40 IU/mg, 30 IU/mg, 20 IU/mg, 15 IU/mg, or 10 IU/mg and an anti-IIa activity less than about 40 IU/mg, 30 IU/mg, 20 IU/mg, 10 IU/mg, 5 IU/mg, 4 IU/mg, or 3 IU/mg); and (c) the preparation has greater than 40% U2SHNS,6S disaccharide residues (e.g., greater than 50%, 60%, 70%, or 80% U2SHNS,6S disaccharide residues). In some embodiments, the preparation has a degree of desulfation less than 40% (e.g., less than 30%, 20%, or 10%).
In a fifth aspect, the invention features a polysaccharide preparation (e.g., a heparin- derived preparation) lacking substantial anticoagulant activity (e.g., having substantially no anticoagulant activity), wherein the preparatiorrmdudes-polv^accharides that include Formula I:
[Uw-HXjy)Z]m~[UG-HX5y5Z]n
wherein U indicates a uronic acid residue and H indicates a hexosamine residue; m and n are integers such that m = 4-16 (e.g., 4-8, 4-9, 4-10, 4-11, 4-12, 4-13, 4-14, or 4-15), and n = 1-4 (e.g., 1-2 or 1-3);
w = -2OS or -2OH; x = -NS or -NAc; y = -3OS or -3OH; z = -60S or -6OH;
wherein the symbol ~ indicates that the units marked m and n are distributed along the polysaccharide chain and are not necessarily in sequence, wherein w, x, y, and z are each the same or different on each unit marked m, and wherein x, y, and z are each the same or different on each unit marked n.
In a sixth aspect, the invention features a polysaccharide preparation (e.g., a heparin- derived preparation) lacking substantial anticoagulant activity (e.g., having substantially noanticoagulant activity) and having antimetastatic activity, wherein the preparation includes polysaccharides that include Formula II:
[Uw-HXjy;Z] m- [UG-HX)y;Z] n- [Uw-HX;y)Z] 0- [UG-HX^2] p- [Uw-HX;yjZ] q
wherein U indicates a uronic acid residue and H indicates a hexosamine residue; wherein m-r are integers such that: m = 0-10; n= 0- 3;
O = O-IO;
P = 0-3; q = 0-10;
w = -2OS or -2OH; x = -NS or -NAc; y = -3OS or -3OH; z = -60S or -6OH;
wherein w, x, y, and z are each the same or different on each unit marked m, n, o, p, or q. In some embodiments, the sum of n + p is less than or equal to 4 (e.g., less than or equal to 3, 2, 1, or 0). In some embodiments, the preparation has a weight average chain molecular weight between 3,500 and 7,000 Da.
Examples of such polysaccharide preparations include chains that include the following:
[Uw-HX;yjZ]m~[UG-Hx y z]n
wherein U indicates a uronic acid residue and H indicates a hexosamine residue, wherein m and n are integers such that m = 6-18, and n = 1 -4, w = -2OS or -2OH, x = -NS or -NAc, y = -3OS or -3OH, z = -60S or -6OH,
wherein the symbol ~ indicates that the units marked m and n are distributed along the polysaccharide chain and are not necessarily in sequence, wherein w, x, y, and z are each the same or different on each unit marked m, and wherein x, y, and z are each the same or different on each unit marked n; and
[Uw-HX)y)Z]m-[UG-HXiy)Z]n-[Uw-HXjyjZ]o-[UG-HX5y)Z]p-[Uw-HX!yiZ]q
wherein U indicates a uronic acid residue and H indicates a hexosamine residue, wherein m-r are integers such that: m = 0-10, n= 0- 3, o = 0-10, p = 0-3, q = 0-10, w = -2OS or -2OH, x = -NS or -NAc, y = -3OS or -3OH, z = -60S or -6OH,
wherein w, x, y, and z are each the same or different on each unit marked m, n, o, p, or q.
Anti-IIa Activity
Polysaccharide preparations are disclosed herein that provide substantially reduced anti- Ha activity, e.g., anti-IIa activity of about 0 to 50 IU/mg, about 0 to 40 IU/mg, about 0 to 30 IU/mg, about 0 to 25 IU/mg, about 0 to 20 IU/mg, about 0 to 10 IU/mg, about 0 to 5 IU/mg, about 5 to 10 IU/mg, about 5 to 15 IU/mg, about 5 to 20 IU/mg. Anti-IIa activity is calculated in International Units of anti- Ha activity per milligram using statistical methods for parallel line assays. The anti-IIa activity levels described herein are measured using the following principle.
Polysaccharide (PS) + ATIII→ [PS • ATIII]
Ha
PS • ATIII→[PS • ATIII • Ha] + Ha (Excess)
Ha (Excess) + Substrate -» Peptide + pNA (measured spectrophotometrically) Anti-factor Ha activity is determined by the sample potentiating effect on antithrombin (ATIII) in the inhibition of thrombin. Thrombin excess can be indirectly spectrophotometrically measured. The anti-factor Ha activity can be measured, e.g., on a Diagnostica Stago analyzer or on an ACL Futura3 Coagulation system, with reagents from Chromogenix (S-2238 substrate, Thrombin (53 nkat/vial), and Antithrombin), or on any equivalent system. Analyzer response is calibrated using the 2nd International Standard for Low Molecular Weight Heparin.
Filed under:
0rphan drug status,
Phase2 drugs Tagged:
M 402,
Necuparanib ![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
Originally posted on AAPS Blog:
 A recent survey and report by PhRMA and Battelle indicates that pharmaceutical executives believe there will be strong growth in R&D and manufacturing but that fewer workers would be needed as “efficiencies” in R&D and manufacturing take hold in the next 10 years.
A recent survey and report by PhRMA and Battelle indicates that pharmaceutical executives believe there will be strong growth in R&D and manufacturing but that fewer workers would be needed as “efficiencies” in R&D and manufacturing take hold in the next 10 years.