
Aplaviroc
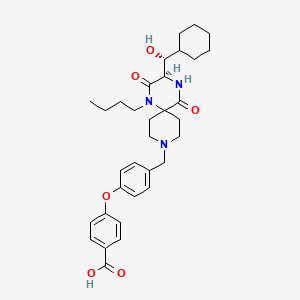
4-(4-{[(3R)-1-butyl-3-[(R)-cyclohexylhydroxymethyl]-2,5-dioxo- 1,4,9-triazaspiro[5.5]undecan-9-yl]methyl}phenoxy)benzoic acid
for the treatment of HIV infection
461023-63-2 of hydrochloride
461443-59-4 (free base)
873140
AK-602
GW-873140
ONO-4128
ono…….innovator
| Ono Pharmaceutical Co., Ltd. |
| Identifiers | |
|---|---|
| CAS number | 461023-63-2  |
| ATC code | None |
| PubChem | CID 3001322 |
| ChemSpider | 2272720  |
| UNII | 98B425P30V  |
| KEGG | D06557  |
| ChEMBL | CHEMBL1255794 |
| Chemical data | |
| Formula | C33H43N3O6 |
| Mol. mass | 577.711 g/mol |
Aplaviroc (INN, codenamed AK602 and GSK-873140) is a CCR5 entry inhibitor developed for the treatment of HIV infection.[1][2] It is developed by GlaxoSmithKline.
In October 2005, all studies of aplaviroc were discontinued due to liver toxicity concerns.[3][4] Some authors have claimed that evidence of poor efficacy may have contributed to termination of the drug’s development;[5] the ASCENT study, one of the discontinued trials, showed aplaviroc to be under-effective in many patients even at high concentrations.[6]
Aplaviroc hydrochloride, an orally-effective, long-acting chemokine CCR5 receptor antagonist, had been under development by Ono and GlaxoSmithKline for the treatment of HIV infection. In early 2006, the companies discontinued development of the antagonist based on reports of elevated liver function test values from clinical studies.
Originally developed at Ono, aplaviroc was licensed to GlaxoSmithKline in 2003 for development, manufacturing and marketing. GlaxoSmithKline also obtained rights to evaluate the agent in non-HIV conditions worldwide with the exception of Japan, South Korea and Taiwan.
A low-molecular-weight compound, aplaviroc prevents HIV viral infection by blocking the binding of the virus to the CCR5 receptor
……………….
WO 2002074770
0r
http://www.google.com/patents/EP1378510A1?cl=en
Reference example 3(3)
- (3R)-1-butyl-2,5-dioxo-3-((1R)-1-hydroxy-1-cyclohexyl)-1,4,9-triazaspiro[5.5]undecane • hydrochloride
-
[0136]
TLC:Rf 0.32 (butanol:acetic acid:water = 4:2:1);
NMR (CD3OD): δ 4.16 (d, J = 2.0 Hz, 1H), 3.95 (m, 1H), 3.70 (m, 1H), 3.52 (m, 1H), 3.37 (m, 1H), 3.28 (m, 1H), 3.22-3.13 (m, 2H), 2.46-1.93 (m, 6H), 1.80-1.64 (m, 5H), 1.48-1.15 (m, 6H), 1.02-0.87 (m, 5H);
Optical rotation:[α]D +1.22 (c 1.04, methanol, 26°C).
Example 9(54)
- (3R)-1-butyl-2,5-dioxo-3-((1R)-1-hydroxy-1-cyclohexylmethyl)-9-(4-(4-carboxyphenyloxy)phenylmethyl)-1,4,9-triazaspiro[5.5]undecane • hydrochloride
-
[0359]
TLC:Rf 0.43(chloroform:methanol = 5:1);
NMR (CD3OD):δ 8.05 (d, J = 9.0 Hz, 2H), 7.61 (d, J = 9.0 Hz, 2H), 7.19 (d, J = 9.0 Hz, 2H), 7.08 (d, J = 9.0 Hz, 2H), 4.38 (s, 2H), 4.17 (d, J = 2.1 Hz, 1H), 4.02 (m, 1H), 3.78 (m, 1H), 3.60-3.40 (m, 3H), 3.30-3.10 (m, 2H), 2.56-1.86 (m, 6H), 1.82-1.60 (m, 5H), 1.52-1.16 (m, 6H), 1.06-0.82 (m, 2H), 0.97 (t, J = 7.2 Hz, 3H).
………………….
http://www.beilstein-journals.org/bjoc/single/articleFullText.htm?publicId=1860-5397-9-265
Owing to the special properties of piperazines (increased solubility and H-bond acceptor capability etc.) it is often considered to be a privileged structure and therefore occurs widely, for instance in GlaxoSmithKlines investigational anti-HIV drug aplaviroc (4.37) which, despite being a promising CCR5 receptor antagonist, was discontinued due to hepatotoxicity concerns. In this compound the spirodiketopiperazine unit (4.35) was designed to mimic a type-1 β-turn (4.36) as present in G-protein coupled receptors (Figure 14) [117].
The synthesis of aplaviroc and its analogues can be accomplished via the use of an Ugi multicomponent reaction (Ugi-MCR) [118]. The procedure involved the condensation of piperidone 4.38 and butylamine (4.39) followed by reaction of the resulting imine with isocyanide 4.41 and interception of the nitrilium intermediate with the amino acid4.40 (Scheme 47) [119]. This sequence was completed by structural rearrangement and acid-mediated ring closure to produce the spirocyclic diketopiperazine 4.43. Following debenzylation this material was subjected to a reductive amination finally affording aplaviroc analogues (Scheme 47).
- 117 Habashita, H.; Kokubo, M.; Hamano, S.; Hamanaka, N.; Toda, M.; Shibayama, S.; Tada, H.; Sagawa, K.; Fukushima, D.; Maeda, K.; Mitsuya, H. J. Med. Chem. 2006, 49, 4140–4152. doi:10.1021/jm060051s
- Dömling, A.; Huang, Y. Synthesis 2008, 2859–2883. doi:10.1055/s-0030-1257906
ref 118 - Nishizawa, R.; Nishiyama, T.; Hisaichi, K.; Matsunaga, N.; Minamoto, C.; Habashita, H.; Takaoka, Y.; Toda, M.; Shibayama, S.; Tada, H.; Sagawa, K.; Fukushima, D.; Maeda, K.; Mitsuya, H.Bioorg. Med. Chem. Lett. 2007, 17, 727–731. doi:10.1016/j.bmcl.2006.10.084
ref 119
| Patent | Submitted | Granted |
|---|---|---|
| Triazaspiro[5.5]undecane derivative and pharmaceutical composition comprising the same as active ingredient [US7262193] | 2005-09-29 | 2007-08-28 |
| Drugs containing triazaspiro[5.5]undecane derivatives as the active ingredient [US7285552] | 2004-06-03 | 2007-10-23 |
| Triazaspiro[5.5]undecane derivatives and drugs containing the same as the active ingredient [US7053090] | 2004-04-29 | 2006-05-30 |
| WO1998031364A1 * | Jan 20, 1998 | Jul 23, 1998 | Timothy Harrison | 3,3-disubstituted piperidines as modulators of chemokine receptor activity |
| WO2000014086A1 * | Jan 21, 1999 | Mar 16, 2000 | Kyowa Hakko Kogyo Kk | Chemokine receptor antagonists and methods of use therefor |
| WO2002074769A1 * | Mar 18, 2002 | Sep 26, 2002 | Kenji Maeda | Drugs containing triazaspiro[5.5]undecane derivatives as the active ingredient |
References
- Maeda, Kenji; Ogata, Hiromi; Harada, Shigeyoshi et al. (2004). “Determination of binding sites of a unique CCR5 inhibitor AK602 / ONO-4128/ GW873140 on human CCR5″ (PDF). Conference on Retroviruses and Opportunistic Infections. Archived from the original on November 3, 2005.
- Nakata, Hirotomo; Maeda, Kenji; Miyakawa, Toshikazu et al. (February 2005). “Potent Anti-R5 Human Immunodeficiency Virus Type 1 Effects of a CCR5 Antagonist, AK602/ONO4128/GW873140, in a Novel Human Peripheral Blood Mononuclear Cell Nonobese Diabetic-SCID, Interleukin-2 Receptor γ-Chain-Knocked-Out AIDS Mouse Model”. Journal of Virology 79 (4): 2087–96.doi:10.1128/jvi.79.4.2087-2096.2005.
- “Aplaviroc (GSK-873,140)”. AIDSmeds.com. October 25, 2005. Retrieved September 5, 2008.[dead link]
- Nichols WG, Steel HM, Bonny T et al. (March 2008). “Hepatotoxicity Observed in Clinical Trials of Aplaviroc (GW873140)”.Antimicrobial Agents and Chemotherapy 52 (3): 858–65. doi:10.1128/aac.00821-07. PMC 2258506. PMID 18070967.
- Moyle, Graeme (December 19, 2006). “The Last Word on Aplaviroc: A CCR5 Antagonist With Poor Efficacy”. The Body.Archived from the original on 6 October 2008. Retrieved September 5, 2008.
- Currier, Judith; Lazzarin, Adriano; Sloan, Louis et al. (2008). “Antiviral activity and safety of aplaviroc with lamivudine/zidovudine in HIV-infected, therapy-naive patients: the ASCENT (CCR102881) study”. Antiviral Therapy (Lond.) 13 (2): 297–306.PMID 18505181.
Further reading
- Horster, S; Goebel, FD (April 2006). “Serious doubts on safety and efficacy of CCR5 antagonists: CCR5 antagonists teeter on a knife-edge”. Infection 34 (2): 110–13. doi:10.1007/s15010-006-6206-1. PMID 16703305.
Filed under: Uncategorized Tagged: Aplaviroc, GlaxoSmithKline


![[1860-5397-9-265-14]](http://www.beilstein-journals.org/bjoc/content/figures/1860-5397-9-265-14.png?max-width=550&background=EEEEEE)
![[1860-5397-9-265-i47]](http://www.beilstein-journals.org/bjoc/content/inline/1860-5397-9-265-i47.png?max-width=550&background=EEEEEE)