FAMOTIDINE
76824-35-6
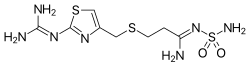
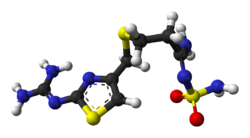
3-(2-Guanidinothiazol-4-ylmethylthio)-N-sulfamoylpropanamidine
MK-208
YM-11170
YM-1170
Histamine H2 Receptor Antagonists
Gastroesophageal Reflux Disease,
Agents forGastric Antisecretory Drugs (GERD)
Astellas Pharma (Innovaator)Launched – 1985
| Systematic (IUPAC) name | |
|---|---|
| 3-[({2-[(diaminomethylidene)amino]-1,3-thiazol-4-yl}methyl)sulfanyl]-N’-sulfamoylpropanimidamide | |
| Clinical data | |
| Trade names | Pepcid |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a687011 |
| Licence data | US FDA:link |
| Pregnancy cat. | |
| Legal status | |
| Routes | Oral (tablet form) |
| Pharmacokinetic data | |
| Bioavailability | 40-45% (Oral)[1] |
| Protein binding | 15-20%[1] |
| Metabolism | hepatic |
| Half-life | 2.5-3.5 hours[1] |
| Excretion | Renal (25-30% unchanged [Oral])[1] |
| Identifiers | |
| CAS number | 76824-35-6  |
| ATC code | A02BA03 |
| PubChem | CID 3325 |
| DrugBank | DB00927 |
| ChemSpider | 3208  |
| UNII | 5QZO15J2Z8  |
| Chemical data | |
| Formula | C8H15N7O2S3 |
| Mol. mass | 337.449 g/mol |
Famotidine is an H2-histamine antagonist that was first launched by Astellas Pharma (formerly Yamanouchi) in Japan in 1985 as an injectable for the treatment of upper gastrointestinal hemorrhage and for the treatment of Zollinger-Ellison syndrome. In 1986, the drug was launched pursuant to a collaboration between Merck Sharp & Dohme and Sigma-Tau for the oral prevention and treatment of gastroesophageal reflux disease (GERD).
Famotidine (INN) /fəˈmɒtɪdiːn/ is a histamine H2-receptor antagonist that inhibits stomach acid production, and it is commonly used in the treatment of peptic ulcer disease (PUD) and gastroesophageal reflux disease (GERD/GORD). It is commonly marketed byJohnson & Johnson/Merck under the trade names Pepcidine and Pepcid and by Astellas under the trade name Gaster. Unlikecimetidine, the first H2 antagonist, famotidine has no effect on the cytochrome P450 enzyme system, and does not appear to interact with other drugs.[2]
Medical use
Certain preparations of famotidine are available over the counter (OTC) in various countries. In the US 20 or more mg, sometimes in combination with a more traditional antacid, are available OTC. Larger doses still require a prescription.

Famotidine is given to surgery patients before operations to prevent postoperative nausea and to reduce the risk of aspiration pneumonitis. Famotidine is also given to some patients taking NSAIDs, to prevent peptic ulcers.[3] It serves as an alternative toproton-pump inhibitors.[4]
It is also given to dogs and cats with acid reflux.
Famotidine has also been used in combination with an H1 antagonist to treat and prevent urticaria caused by an acute allergic reaction.[5]
Side-effects
Side-effects are associated with famotidine use. In clinical trials, the most common adverse effects were headache, dizziness, andconstipation or diarrhea.[6]
History
Famotidine was developed by Yamanouchi Pharmaceutical Co.[7] It was licensed in the mid-80s by Merck & Co.[8] and is marketed by a joint venture between Merck and Johnson & Johnson. The imidazole-ring of cimetidine was replaced with a 2-guanidinothiazole ring. Famotidine proved to be 30 times more active than cimetidine.[citation needed]
It was first marketed in 1981. Pepcid RPD orally-disintegrating tablets were released in 1999. Generic preparations became available in 2001, e.g.Fluxid (Schwarz) or Quamatel (Gedeon Richter Ltd.).
In the United States and Canada, a product called Pepcid Complete, which combines famotidine with an antacid in a chewable tablet to quickly relieve the symptoms of excess stomach acid, is available. In the UK, this product is known as Pepcidtwo.
Famotidine suffers from poor bioavailability (50%), as it is poorly soluble in the low pH of the stomach. Famotidine used in combination with antacids promotes local delivery of these drugs to the receptor of the parietal cell wall. Therefore, researchers are developing innovative formulations of tablets, such as gastroretentive drug delivery systems. Such tablets are retained in the stomach for a longer period of time, thereby improving the bioavailability of drugs. Local delivery also increases bioavailability at the stomach wall receptor site and increases the efficacy of drugs to reduce acid secretion.[9]
Research
Famotidine has been investigated as an adjunct in treatment-resistant schizophrenia. In one trial it caused a 10% reduction in schizophrenic symptom severity in treatment-resistant patients.[10]
Famotidine is also indicated in the treatment of duodenal and benign gastric ulcers, for the prevention of relapse of duodenal ulceration, for the treatment of gastric mucosal lesions associated with acute gastritis and acute exacerbation of chronic gastritis, for the treatment of heartburn associated with acid indigestion and sour stomach, for the prevention of meal-induced heartburn, and for the treatment of reflux esophagitis due to GERD, including ulcerative disease as diagnosed by endoscopy. The drug is currently marketed in tablet, film-coated tablet, orally-disintegrating tablet, powder, lyophilized powder for injection solution and injectable formulations. The compound had been in development for the treatment of non-erosive reflux disease (NERD), however, Astellas Pharma discontinued development for this indication in 2007.

Famotidine was developed by replacing the imidazole ring of GlaxoSmithKline’s cimetidine with a 2-guanidinothiazole ring, a modification proven to increase the activity of the drug 30-fold. Famotidine competitively inhibits the action of histamine at the histamine H2 receptors of the parietal cells. It suppresses the normal secretion of acid by parietal cells and the meal-stimulated secretion of acid by two mechanisms: by blocking histamine released by enterochromaffin-like (ECL) cells in the stomach from binding to H2 receptors and stimulating acid secretion, and by reducing the effect that other compounds (such as gastrin, pentagastrin, caffeine, insulin and acetylcholine) have on the promotion of acid secretion due to H2 receptor blockade.
Famotidine was originally developed at Astellas Pharma. It was subsequently licensed in the U.S. to Merck & Co., known outside the U.S. and Canada as Merck Sharp & Dohme. In 2007, Salix acquired the U.S. rights to famotidine oral solution (Pepcid[R]) for the treatment of GERD and peptic ulcer. Sigma-Tau holds rights to the drug and is responsible for marketing activities in Italy. Famotidine is sold in over 110 countries worldwide, including France, Germany, Italy, Japan, the U.S. and the U.K.

……………………………..
US 4283408
http://www.google.co.in/patents/US4283408


The reaction ot S-(2-aminothiazol-4-ylmethyl)isothiourea (I) with 3-chloropropionitrile (II) by means of NaOH in ethanol – water gives 3-(2-aminothiazol-4-ylmethylthio)propionitrile (III), which is condensed with benzoyl isothiocyanate (IV) in refluxing acetone to afford 3-[2-(3-benzoylthioureido)thiazol-4-ylmethylthio]propionitrile (V). The hydrolysis of (V) with K2CO3 in acetone – methanol – water yields 3-(2-thioureidothiazonl-4-ylmethylthio)propionitrile (VI), which by methylation with methyl iodide in refluxing ethanol is converted into 3-[2-(S-methylisothioureido)thiazol-4-ylmethylthio]propionitrile hydroiodide (VII). The reaction of (VII) with NH3 and NH4Cl in methanol at 90 C in a pressure vessel affords 3-(2-guanidinothiazol-4-ylmethylthio)propionitrile (VIII), which by partial alcoholysis with methanol by means of dry HCl in CHCl3 is converted into methyl 3-(2-guanidinothiazol-4-ylmethylthio)propionimidate (IX). Finally, this compound is treated with sulfamide in refluxing methanol.
Drugs Fut 1983, 8, 1, 14
US 4283408
DOS 2 951 675 (Yamanouchi; appl. 21.12.1979; J-prior. 2.8.1979).
DOS 3 008 056 (Yamanouchi; appl. 3.3.1980; J-prior. 6.3.1979, 23.6.1979).
GB 2 052 478 (Yamanouchi; appl. 6.3.1980; J-prior. 6.3.1979, 23.6.1979).
GB 2 055 800 (Yamanouchi; appl. 20.12.1979; J-prior. 2.8.1979).
synthesis of S-[2-aminothiazol-4-ylmethyl]isothiourea:
Spragne, J.M.; Lund, A.H.; Ziegler, C.: J. Am. Chem. Soc. (JACSAT) 68, 2155 (1946).
FT IR OF FAMOTIDINE

http://link.springer.com/article/10.1007%2Fs00216-011-5599-6
20121204-12383-10xabe9.png?1354675130)
[1H,13C] 2D NMR Spectrum
………………………..
UV – range
IR – spectrum
…………..
Synthesis pathway
Trade names
| Country | Trade name | Manufacturer |
|---|---|---|
| Germany | Fadul | Hexal |
| Famobeta | betapharm | |
| Famonerton | Dolorgiet | |
| Pepdul | TEOFARMA | |
| various generic drugs | ||
| France | Peptsidak | McNeil |
| Peptsidduo | McNeil | |
| Pepdin | Merck Sharp & Dohme-Chibret | |
| Great Britain | Peptsid | Merck Sharp & Dohme |
| Italy | Famoudou | Sigma-Tau |
| Gastridin | Merck Sharp & Dohme | |
| Motiaks | Neopharmed | |
| Japan | Gaster | Astellas |
| United States | Peptsid | Merck, 1986 |
| - “- | Johnson & Johnson; Merck | |
| Ukraine | Ulfamid | Krka, dd, Novo mesto, Slovenia |
| Kvamatel | JSC “Gedeon Richter”, Hungary | |
| Famasan | ABM. MED. CA AT Prague, Czech Republic | |
| FamodinGeksal | Salyutas Pharma GmbH, Germany, venture hexane AG, Germany | |
| various generic drugs | ||
Formulations
-
ampoule 10 mg, 20 mg;
-
Tablets coated with 10 mg, 20 mg, 40 mg;
-
oral suspension, 40 mg / 5 ml;
-
2% powder, 10%;
-
10 mg tablets, 20 mg;
-
vials (lyophilisate) 20 mg
Reference for above
-
DOS 2,951,675 (Yamanouchi; appl. 21.12.1979; J-prior. 2.8.1979).
-
DOS 3,008,056 (Yamanouchi; appl. 3.3.1980; J-prior. 6.3.1979, 23.6.1979).
-
GB 2052478 (Yamanouchi; appl. 6.3.1980; J-prior. 6.3.1979, 23.6.1979).
-
GB 2055800 (Yamanouchi; appl. 20.12.1979; J-prior. 2.8.1979).
-
US 4,283,408 (Yamanouchi; 11.8.1981; J-prior. 2.8.1979).
References
- Truven Health Analytics, Inc. DRUGDEX® System (Internet) [cited 2013 Oct 10]. Greenwood Village, CO: Thomsen Healthcare; 2013.
- Humphries TJ, Merritt GJ (August 1999). “Review article: drug interactions with agents used to treat acid-related diseases” (pdf). Aliment. Pharmacol. Ther. 13 (Suppl 3): 18–26.doi:10.1046/j.1365-2036.1999.00021.x. PMID 10491725.
- “Horizon Pharma, Inc. Announces FDA Approval of DUEXIS(R) for the Relief of the Signs and Symptoms of Rheumatoid Arthritis and Osteoarthritis and to Decrease the Risk of Developing Upper Gastrointestinal Ulcers” (Press release). Horizon Pharma. 2011-04-25.
- Brauser D (Jul 13, 2009). “Famotidine May Prevent Peptic Ulcers, Esophagitis in Patients Taking Low-Dose Aspirin”. Medscape.
- Fogg TB, Semple D (29 November 2007). “Combination therapy with H2 and H1 antihistamines in acute, non compromising allergic reactions”. BestBets. Manchester, England: Manchester Royal Infirmary. Retrieved 26 April 2011.
- “Pepcid Side Effects & Drug Interactions”. RxList.com. 2008. Retrieved 2008-07-31.
- US patent 4283408, HIRATA YASUFUMI; YANAGISAWA ISAO; ISHII YOSHIO; TSUKAMOTO SHINICHI; ITO NORIKI; ISOMURA YASUO; TAKEDA MASAAKI, “Guanidinothiazole compounds, process for preparation and gastric inhibiting compositions containing them”, issued 1981-08-11
- “Sankyo Pharma”. Skyscape Mediwire. 2002. Retrieved 2009-10-30.[dead link]
- “Formulation and Evaluation of Gastroretentive Floating Tablets of Famotidine”. Farmavita.Net. 2008. Retrieved 2009-01-30.
- Meskanen, K; Ekelund, H; Laitinen, J; Neuvonen, PJ; Haukka, J; Panula, P; Ekelund, J (August 2013). “A randomized clinical trial of histamine 2 receptor antagonism in treatment-resistant schizophrenia.”. Journal of Clinical Psychopharmacology 33 (4): 472–478. doi:10.1097/JCP.0b013e3182970490. PMID 23764683.
Filed under: GENERIC DRUG Tagged: FAMOTIDINE, Gastroesophageal Reflux Disease, H2 Receptor Antagonists, peptic ulcer disease, Yamanouchi Pharmaceutical










