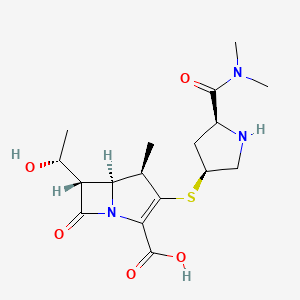
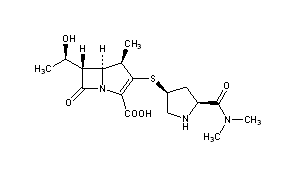
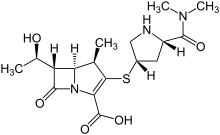
Meropenem
CAS number96036-03-2
IUPAC Name(4R,5S,6S)-3-{[(3S,5S)-5-(dimethylcarbamoyl)pyrrolidin-3-yl]sulfanyl}-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid
WeightAverage: 383.463
Monoisotopic: 383.151491615
Chemical FormulaC17H25N3O5S
- Antibiotic SM 7338
- ICI 194660
- SM 7338
CAS Registry Number: 96036-03-2
CAS Name: (4R,5S,6S)-3-[[(3S,5S)-5-[(Dimethylamino)carbonyl]-3-pyrrolidinyl]thio]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid
Additional Names: (1R,5S,6S)-2-[(3S,5S)-5-(dimethylaminocarbonyl)pyrrolidin-3-ylthio]-6-[(R)-1-hydroxyethyl]-1-methylcarbapen-2-em-3-carboxylic acid
Molecular Formula: C17H25N3O5S
Molecular Weight: 383.46
Percent Composition: C 53.25%, H 6.57%, N 10.96%, O 20.86%, S 8.36%
Literature References: Carbapenem antibiotic. Prepn: M. Sunagawa et al.,EP126587; M. Sunagawa, US4943569 (1984, 1990 both to Sumitomo).
Structure-activity study: M. Sunagawa et al.,J. Antibiot.43, 519 (1990).Crystal structure: K. Yanagi et al.,Acta Crystallogr.C48, 1737 (1992).HPLC determn in serum and bronchial secretions: M. Ehrlich et al., J. Chromatogr. B751, 357 (2001). Pharmacokinetics: R. Wise et al.,Antimicrob. Agents Chemother.34, 1515 (1990).Series of articles on antimicrobial activity, metabolism: J. Antimicrob. Chemother.24, Suppl. A, 1-320 (1989); and clinical performance: ibid.36, Suppl. A, 1-223 (1995).Review of clinical experience in intensive care: M. Hurst, H. M. Lamb, Drugs59, 653-680 (2000).
Derivative Type: Trihydrate
CAS Registry Number: 119478-56-7
Manufacturers’ Codes: ICI-194660; SM-7338
Trademarks: Meronem (AstraZeneca); Meropen (Sumitomo); Merrem (AstraZeneca)
Properties: White to pale yellow crystalline powder. Sparingly sol in water; very slightly sol in hydrated ethanol. Practically insol in acetone, ether.
Therap-Cat: Antibacterial.
Keywords: Antibacterial (Antibiotics); ?Lactams; Carbapenems.
Product Ingredients
| INGREDIENT | UNII | CAS | INCHI KEY |
|---|---|---|---|
| Meropenem sodium | Not Available | 211238-34-5 | UBQRNADYCUXRBD-NACOAMSHSA-N |
| Meropenem trihydrate | FV9J3JU8B1 | 119478-56-7 | CTUAQTBUVLKNDJ-OBZXMJSBSA-N |
International/Other BrandsAronem (ACI) / Aropen (Aristopharma) / Carbanem (Sanofi-Aventis) / Erope (Lincoln) / Fulspec (Acme) / I-penam (Incepta) / Merenz (Admac) / Merofit (FHC) / Meronem (AstraZeneca) / Meronis (Neiss) / Meropen (Swiss Parenterals) / Merotec (Zuventus) / Merrem I.V. (AstraZeneca) / Monan (AstraZeneca) / Ropenem (Drug International) / Zeropenem (Sanofi-Aventis)
Synthesis Reference
Yoon Seok Song, Sung Woo Park, Yeon Jung Yoon, Hee Kyoon Yoon, Seong Cheol Moon, Byung Goo Lee, Soo Jin Choi, Sun Ah Jun, “METHOD FOR PREPARING MEROPENEM USING ZINC POWDER.” U.S. Patent US20120065392, issued March 15, 2012.
SYN
Carbapenem antibiotic. Prepn: M. Sunagawa et al., EP 126587; M. Sunagawa, US 4943569 (1984, 1990 both to Sumitomo). Structure-activity study: M. Sunagawa et al., J. Antibiot. 43, 519 (1990).

SYN
https://patents.google.com/patent/WO2012062035A1/enCarbapenem, a type of β-lactam antibiotic, is known for its broad spectrum of antibacterial activity and strong antibacterial activity, such as meropenem (Me r0 p e nem), imine South (Imipenem) and Biabenem, etc., play an important role in the cure of severe infections.

Meropenem Imipenem For the synthetic methods of the Peinan type, the previous studies have mainly synthesized the corresponding Peinan side chain compound and the parent nucleus MAP, respectively, and then condensed and removed the protecting group to obtain the Peinan product. Such as US patentsUSP4933333, starting from 4-acetoxyazetidinone (4AA), obtained a matrix MAP after several steps of reaction. The mother nucleus is then condensed and deprotected from the side chain to obtain meropenem. However, this method is cumbersome, the synthesis step is long, and the total yield is low, and the noble metal catalyst is inevitably used in the synthesis of the compound (9).

MAP (10) Meropenem The Chinese invention patent document CN200810142137.5 has introduced a method for synthesizing meropenem.

(XII) (I)(TBD S = Si (CH 3 ) 2 C (CH 3) 3; PNB = p-N0 2 -C 6 H 4 CH 2; PNZ = 2 -C 6 H 4 CH 2 OCO N0 p-) This method of Scheme Short, easy to operate, easy to get raw materials, but there are some areas for improvement.

Example 11) (3R, 4S)-3-[(R)-l-(tert-butyldimethylsilyloxy)ethyl]-4-[(2,S, 4’R)- 1- (allyl Synthesis of oxycarbonylxiaodimethylaminocarbonylpyrrolidinothio]-2-azetidinone (II) In a 500 ml reaction flask, add 22.6 g (0.075 mol) of (3S,4S)-3-[( R) l-(tert-Butyldimethylsilyloxy)ethyl]-4-[(R)-1-carbonylethyl]-2-azetidinone (IV), 17.1 g (0.083 mol) Dicyclohexylcarbodiimide (DCC) in 100 ml of acetone and 0.76 g of 4-dimethylaminopyridine (DMAP), 20.3 g (0.078 mol) of (2S, 4R)-2-dimethylamine was added dropwise with stirring. A solution of carbonyl-4-mercapto (i-propoxycarbonyl)pyrrolidine (V) in 125 ml of acetone was reacted at room temperature for 14 hours. Filtration, collecting the filtrate, concentrating, adding 200 ml of toluene thereto, using 200 ml of a 5 % acetic acid solution, 200 ml of a saturated sodium hydrogencarbonate solution and 150 ml of saturation Washed with brine, dried over anhydrous magnesium sulfate and evaporated to dryness <mjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjj 4-[(2,8, 4, ) small (propoxycarbonyl dimethyl dimethylaminocarbonyl)pyrrolidinyl]-2-azetidinone (II), directly without further treatment Invest in the next step.1H-NMR (400 MHz, CDC 13): </ RTI> <RTIgt; m), 2.816-2.849 (lH, s), 2.935-2.953 (3H, m), 3.027-079 (3H, d), 3.378-3.401 (lH, m), 3.792-3.796 (1H, d), 3.807- 3.953 (lH, m), 4.042-4.160 (3H, m), 4.492-4.570 (2H, m), 4.670-4.739 (lH, m), 5.164-5.295 (1H, m), 5.807-5.921 (lH, m ), 6.214(1H, s). Example 22) (31,48)-3-[(1 )-1-(tert-butyldimethylsilyloxy)ethyl]-4-[(2,8,4,1 )- 1- (allyl Synthesis of oxycarbonyl-1-dimethylaminocarbonylpyrrolidinothio]-1-(zincpropoxyl)-2-azetidinone (III) In a 1000 ml reaction flask, add 34.8 g (0.064) Mol) (3R, 4S)-3-[(R)-l-(tert-butyldimethylsilyloxy)ethyl]-4-[(2,S, 4,R)-1-(allyl Oxycarbonyl-1-pyrimidinylcarbonyl)pyrrolidinylthio]-2-azetidinone (11), 15.0 ml of triethylamine and 350 ml of toluene, control temperature below -10 °C, add 18.9 g (0.128 mol) p-nitrobenzyl chloroacetate (VI), heated to 0 ° C (-20 ° 5 ° C can be) reaction l ~ 3h. Then slowly add 250 ml of ice water and stir for 10 min. The layers were static and the organic phase was washed three times with saturated sodium bicarbonate solution, 200 ml each time. Dry over anhydrous magnesium sulfate, filtered, and evaporated to dryness to give white crystals, 4,7g (0.0622mol, yield 97.3%) (3R, 4S)-3-[(R) small (tert-butyldimethylsilyloxy)ethyl ]-4-[(2,S, 4,R)-1-(allyloxycarbonyldimethyldimethylaminocarbonyl)pyrrolidinylsulfur]sodium (sweetoxypropanoyl)-2-azetidinone (III), the product was directly put into the next step without further purification.Mp: 33-34 °C1H-NMR (300 MHz, CDC 13):0.819(9H, s), 1.167(3H, d), 1.188(4H, d), 1.693(5H, s), 1.850-1.926(1H, m), 2.631-2.700(1H, m), 2.941-2.960( 3H,d), 3.029-3.080(3H,d), 3.357-3.433(lH, m), 3.506-3.545(2H, m), 3.918-3.968(1H, m), 4.054-4.123 (2H, m), 4.270-4.291(lH, m), 4.391(lH,s), 4.518-4.568(2H, m), 4.588-4.779(3H, m), 5.178-5.416(3H, m), 5.861-5.982(2H,m ). Example 33) (5R,6S,8R,2’S, 4,S)-[(R)-1-(tert-butyldimethylsilyloxy)ethyl]-3-[4-(1-allyloxycarbonyl) -1- dimethylaminocarbonylpyrrolidinothio]-6-(1-allyloxycarbonylethoxy)-1-azabicyclo[3.2.0]-hept-2-en-7-one- Synthesis of 2-carboxylate In a 500 ml reaction flask, 40; 7 g (0.0622 mol) of (3R, 4S)-3-[(R)-l-(tert-butyldimethylsilyloxy) was added. Ethyl]-4-[(2,S,4,R)-1-(indolyloxycarbonyl-1-dimethylaminocarbonyl)pyrrolidinylsulfate]small (sweetoxypropanoyl)-2-nitrogen Heterocyclic butanone (III) and 150 ml of toluene, 22 ml of trimethyl phosphite (furrowing lg of hydroquinone) were added under nitrogen. After reacting at 60 ° C for 16 hours, the solvent was evaporated under reduced pressure. It was recrystallized by adding 300 ml of ethyl acetate, and the solid was collected, and vacuum-dried at 40 ° C to obtain 32.8 g (0.0528 mol, yield: 85.0%) (5R, 6S, 8R, 2’S, 4,S)-[(R)- 1-(tert-Butyldimethylsilyloxy)ethyl]-3-[4-(1-allyloxycarbonyl-1-dimethylaminocarbonyl)pyrrolidinyl] -6-(1-ene Propoxycarbonyl ethoxy) small azabicyclo[3.2.0]-hept-2-en-7-one-2-carboxylate (oxime).1H-NMR (300 MHz, CDC 13):0.82(9H, s), 1.24(6H, d), 1.26(3H, s), 1.36(3H, s), 1.94(1H, m), 2.69(1 H, m), 2.97-3.11(6H, m ), 3.15-3.74(4H, m), 4.35(2H,m), 4.37-4.67(5H, m), 5.24-5.28(4H, m), 5.84(1H, m). Example 44) (5R, 6S, 8R, 2, S, 4’S)-[(R)小(hydroxy)ethyl]-3-[4-(1-allyloxycarbonylsuccinylcarbonyl)pyrrolidinyl Synthesis of thio]-6-(1-allyloxycarbonylethoxy)-1-azabicyclo[3.2.0]-hept-2-en-7-one-2-carboxylate (Vffl) at room temperature , in a 2000ml reaction flask, add 32.8g (0.0528mol) (5R,6S,8R,2’S,4,S)-[(R)-1-(tert-butyldimethylsilyloxy)ethyl] 3-[4-(1-allyloxycarbonyl-1-dimethylaminocarbonyl)pyrrolidinyl]-6-(1-indolyloxycarbonylethoxy)-1-azabicyclo[3.2.0 -Hept-2-ene-7-one-2-carboxylate (W), 27.4 ml of acetic acid, 41.3 g of fluorohydrogenamine and 1000 ml of dichloromethane, stirred at room temperature for 48 h. After completion of the reaction, 500 ml of a saturated aqueous solution of sodium hydrogencarbonate was added to the reaction mixture, and the mixture was stirred for 10 minutes, and the methylene chloride layer was separated and dried over anhydrous magnesium sulfate to give a white solid (26.2 g (0.0517 mol, yield 98.0). %) (5R, 6S, 8R, 2’S, 4’S)-[(R)小(hydroxy)ethyl]-3-[4-(1-allyloxycarbonylsuccinylcarbonyl)pyr Rhodium thio] -6-(l-allyloxycarbonylethoxy)-1-azabicyclo[3. 2. 0]-hept-2-en-7-one-2-carboxylate (ring The product was directly charged to the next step without further purification.1H-NMR (300 MHz, CDC 13):1.26(3H, s), 1.36(3H, s), 1.94(1H, m), 2.67(1H, m), 2.97-3.11(6H, m), 3.2-3.7(4H, m) ; 4.25(2H, m), 4.47-4.87 (5H, m), 5.15-5.50 (4H, m), 5.94 (2H, m). Example 55) (5R,6S,8R,2,S,4,S)-3-[4-dimethylaminocarbonyl)pyrrolidinyl]-6-(l-hydroxyethyl)-1-aza Synthesis of bicyclo[3.2.0]-hept-2-en-7-one-2-carboxylate (I) To the reaction flask, 26.2 g (0.0517 mol) (5R, 6S, 8R, 2’S, 4’S) was added. – [(R)-l-(hydroxy)ethyl]-3-[4-(1-allyloxycarbonyl-1-dimethylaminocarbonyl)pyrrolidinyl] -6-(1-allyloxy Carbonyl ethoxy)-1-azabicyclo[3. 2. 0]-hept-2-en-7-one-2-carboxylate (VDI), 21.3 g (0.152 mol) dimethylcyclohexane The ketone and 550 ml of ethyl acetate were heated to 30 ° C, and a solution of 1.0 g (0.865 mmol) of tetratriphenylphosphine palladium in 150 ml of dichloromethane was added dropwise thereto, and the mixture was reacted at room temperature for 3 h under nitrogen atmosphere. After adding 300 ml of water to the reaction mixture, the aqueous layer was separated, the aqueous layer was washed with ethyl acetate, and then, 500 ml of tetrahydrofuran was added dropwise with stirring in an ice bath, and the crystals were stirred, and the crystals were collected and dried in vacuo to give pale yellow crystals of 13.4 g (0.0352 md, Yield 68.1%) (5R,6S,8R,2,S,4,S)-3-[4-(2-dimethylaminocarbonyl)pyrrolidinylthio]-6-(1-hydroxyethyl) 1-Azabicyclo[3.2.0]-hept-2-en-7-one-2-carboxylic acid trihydrate (I)-Meropectin.IR max KBr cm- 1 : 1755, 1627, 1393, 1252, 1130NMR (D20, 300Hz): 1.25 (3H, d), 1.81-1.96 (1H, m), 2.96 (3H, s), 3.03 (3H, s), 3.14-3.20 (3H, m), 3.31-3.41 (2H, m), 3.62- 3.72 (1H, m), 3.90-4.00 (1H, m), 4.14-4.26 (2H, m), 4.63 (1H, t). Example 6 6) (5R,6S,8R,2’S,4’S)-3-[4-(2-Dimethylaminocarbonyl)pyrrolidinylthio]-6-(l-hydroxyethyl)-1-azabicyclo[ Synthesis of 3.2.0]-hept-2-en-7-one-2-carboxylate (I)21.3 g (0.152 mol) of dimethylcyclohexanedione in Example 5 was replaced with 45.1 g (0.155 mol) of tributyltin hydride, and 0.125 g (0.108 mmol) of tetrakistriphenylphosphine palladium was added dropwise, and the other amount was added. And the same method, the obtained 16.2g (0.0426mol, 82.5%) (5R,6S,8R,2’S,4’S)-3-[4-(2-dimethylaminocarbonyl)pyrrolidinyl Sulfur]-6-(l-hydroxyethyl)-1-azabicyclo[3.2.0]-hept-2-en-7-one-2-carboxylic acid trihydrate (1) ~ meropenem. Example 7 7) (5R,6S,8R,2,S,4,S)-3-[4-(2-dimethylaminocarbonyl)pyrrolidinyl]-6-(1-hydroxyethyl)-1- Synthesis of azabicyclo[3.2.0]-hept-2-en-7-one-2-carboxylate (I) To the reaction flask, 26.2 g (0.0517 mol) of (5R, 6S, 8R, 2, S, 4’S)-[(R)-l-(hydroxy)ethyl]-3-[4-(1-allyl was added) Oxycarbonyl-1-ylaminocarbonylcarbonylpyrrolidinothio]-6-(1-allyloxycarbonylethoxy)azaabicyclo[3. 2.]-hept-2-ene-7- Ketone-2-carboxylate 01), 6.0 g (0.0387 mol) of N, N-dimethylbarbituric acid and 500 ml of dichloromethane, and 6.0 g (5.2 mmol) of tetratriphenylphosphine was added dropwise thereto. A solution of palladium in 100 ml of dichloromethane was reacted at room temperature for 5 h under nitrogen. After adding 300 ml of water to the reaction mixture, the aqueous layer was separated, and the aqueous layer was washed with ethyl acetate. THF was evaporated and evaporated, and the crystals were evaporated, and crystals were collected, and the crystals were dried in vacuo to give 15.7 g (0.0413 mol, yield: 80.1%). 5R, 6S, 8R, 2,S,4,S) – 3-[4-(2-Dimethylaminocarbonyl)pyrrolidinylthio]-6-(1-hydroxyethyl)-1-azabicyclo [3. 2. 0] -Hept-2-ene-7-keto-2-carboxylic acid trihydrate (I)-Meropectin.
ClaimsHide Dependent
Rights requesta synthetic method of meropenem, characterized in that the specific reaction route of the synthetic method

The reaction steps are as follows:1) The compound of the formula (IV) and the compound of the formula (V) are dissolved in an organic solvent and then subjected to a condensation reaction to obtain a compound of the formula (Π), the reaction time is 2 to 24 hours, and the reaction temperature is 0 to 40 ° C. ;2) The compound of the formula (Π) and the compound of the formula (VI) are dissolved in toluene, ethyl acetate or tetrahydrofuran and reacted with a base to form a compound of the formula (III), and the reaction time is ! ~ 3 hours, the reaction temperature is -20~5 °C;3) The compound of the formula (III) is dissolved in cyclohexanyl, n-glyoxime, n-octyl, toluene or xylene, and a Wittig ring-closing reaction is carried out under the action of an organophosphorus reagent to obtain a compound of the formula (VD), the organophosphorus reagent Is triphenylphosphine, tri-n-butylphosphine, triethyl phosphite or trimethyl phosphite;4) The compound of the formula (VII) is dissolved in methanol, tetrahydrofuran, acetone, n-pentane, n-hexane, diethyl ether, acetonitrile, dichloromethane, chloroform or ethyl acetate to hydrolyze the silyl ether bond under the action of an acid to obtain a formula (W). a compound; the acid is dilute hydrochloric acid, hydrofluoric acid, tetrabutylammonium fluoride, benzyltributylammonium fluoride, hydrofluoric hinge or vinegar The acid, the molar ratio of the acid to the compound of the formula is 5 to 15: 1; the temperature of the hydrolysis reaction is 0 to 40 ° C, and the reaction time is 8 to 24 hours;5) a compound of the formula
dissolved in one or more of methanol, ethanol, tert-butanol, isobutanol, isopropanol, tetrahydrofuran, dioxanthene, acetone, dichloromethane, chloroform and water After the solvent is formed, the allylic group is hydrogenated by a palladium catalyst to obtain the target product (1). The molar ratio of the palladium catalyst to the compound of the formula 1) is 0.0001 to 0.5:1; the reaction temperature is 0 to 40 ° C. , the reaction time is 2~24h.2. A method for synthesizing meropenem according to claim 1, wherein the molar ratio of the compound of the formula (IV) to the compound of the formula (V) is 1.05 to 1.0: 1, the condensing agent and The molar ratio of the compound of the formula (IV) is 1.50 to 1.05:1.The method for synthesizing meropenem according to claim 1 or 2, wherein the condensing agent is a carbodiimide reagent or hydrazine, Ν’-carbonyldiimidazole; and the organic solvent is acetone. , acetonitrile, toluene, tetrahydrofuran, chloroform or dimethylformamide.The method for synthesizing meropenem according to claim 1, wherein the molar ratio of the compound of the formula (VI) to the compound of the formula (VI) is from 1.5 to 2.5:1, the base and the The molar ratio of the compound of the formula (VI) is from 1.2 to 2:1.The method for synthesizing meropenem according to claim 1, wherein the molar ratio of the organophosphorus reagent to the compound of formula (III) in step 3) is 2-8: 1; The reaction temperature is 25 to 100 £ ^, and the reaction time is 10 to 24 hours.The method for synthesizing meropenem according to claim 3, wherein the carbodiimide reagent is dicyclohexylcarbodiimide, diisopropylcarbodiimide or 1-( 3-dimethylaminopropyl)-3-ethylcarbodiimide.7. A method for synthesizing meropenem according to claim 1, wherein the base in step 2) is an inorganic base or an organic base; when it is an inorganic base, it is sodium hydroxide, sodium carbonate or Sodium bicarbonate; when it is an organic base, it is pyridine, triethylamine, diisopropylethylamine or 2,6-lutidine.The method for synthesizing meropenem according to claim 1, wherein the palladium catalyst is palladium acetate, palladium chloride, palladium nitrate, bistriphenylphosphine palladium chloride or tetrakistriphenylphosphine. palladium.9. A method for synthesizing meropenem according to claim 1, wherein the protecting group acceptor in step 5) is morpholine, dimethylcyclohexanedione, tributyltin hydride, N, N-dimethylbarbituric acid, -ethylhexanoic acid or hexanoic acid.
SYN

Reference: Nadenik, Peter; Storm, Ole; Kremminger, Peter. Meropenem intermediate in crystalline form. WO 2005118586. (Assignee Sandoz AG, Switz)
SYN 2

Reference: Nishino, Keita; Koga, Teruyoshi. Improved process for producing carbapenem compound. WO 2007111328. (Assignee Kaneka Corporation, Japan)
SYN 3

Reference: Manca, Antonio; Monguzzi, Riccardo Ambrogio. Process for synthesizing carbapenem using Raney nickel. EP 2141167. (Assignee ACS Dobfar S.p.A., Italy)
SYN 4

Reference: Tseng, Wei-Hong; Chang, Wen-Hsin; Chang, Chia-Mao; Yeh, Chia-Wei; Kuo, Yuan-Liang. Improved process for the preparation of carbapenem using carbapenem intermediates and recovery of carbapenem. EP 2388261. (Assignee Savior Lifetec Corp., Taiwan)
STR5

Reference: Gnanaprakasam, Andrew; Ganapathy, Veeramani; Syed Ibrahim, Shahul Hameed; Karthikeyan, Murugesan; Sivasamy, Thangavel; Michael, Sekar Jeyaraj; Arulmoli, Thangavel; Das, Gautam Kumar. Preparation of meropenem trihydrate. WO 2012160576. (Assignee Sequent Anti Biotics Private Limited, India)
SYN 6

Reference: Gnanprakasam, Andrew; Ganapathy, Veeramani; Syed Ibrahim, Shahul Hameed; Karthikeyan, Murugesan; Sivasamy, Thangavel; Sekar, Jeyaraj; Arulmoli, Thangavel. Preparation of meropenem trihydrate. IN 2011CH01780. (Assignee Sequent Scientific Limited, India)
SYN7

Reference: Senthikumar, Udayampalayam Palanisamy; Sureshkumar, Kanagaraj; Babu, Kommoju Nagesh; Sudhan, Henry Syril; Kamaraj, Ponraj Pravin; Suresh, Thangaiyan. An improved process for the preparation of carbapenem antibiotic. WO 2013150550. (Assignee Orchid Chemicals & Pharmaceuticals Limited, India)
SYN 8

Reference: Ong, Winston Zapanta; Nowak, Pawel Wojciech; Kim, Jinsoo; Enlow, Elizabeth M.; Bourassa, James; Cu, Yen; Popov, Alexey; Chen, Hongming. Meropenem derivatives and uses thereof. WO 2014144285. (Assignee Kala Pharmaceuticals, Inc., USA)
SYN9

Reference: Cookson, James; McNair, Robert John; Satoskar, Deepak Vasant. Preparation of a carbapenem antibiotic by hydrogenation in the presence of a heterogeneous catalyst. WO 2015145161. (Assignee Johnson Matthey Public Limited Company, UK)
SYN 10

Reference: Gruenewald, Elena; Weidlich, Stephan; Jantke, Ralf. Process for the deprotection of a carbapenem by heterogeneous catalytic hydrogenation with hydrogen in the presence of an organic amine. WO 2018010974. (Assignee Evonik Degussa GmbH, Germany)
SYN 11


Some improvements in total synthesis of meropenem; Hu, Lai-Xing; Liu, Jun; Jin, Jie; Zhongguo Yiyao Gongye Zazhi; Volume 31; Issue 7; Pages 290-292; Journal; 2000
synhttps://www.researchgate.net/figure/Synthesis-of-MRPD-starting-from-meropenem_fig9_283306781

//////////////////////////

join me on Linkedin
Anthony Melvin Crasto Ph.D – India | LinkedIn
join me on Researchgate
RESEARCHGATE

join me on Facebook
Anthony Melvin Crasto Dr. | Facebook
join me on twitter
Anthony Melvin Crasto Dr. | twitter
+919321316780 call whatsaapp
EMAIL. amcrasto@amcrasto
/////////////////////////////////////////////////////////////////////////////////////////////////////
Meropenem is an ultra-broad spectrum injectable antibiotic used to treat a wide variety of infections, including meningitis and pneumonia. It is a beta-lactam and belongs to the subgroup of carbapenem, similar to imipenem and ertapenem. Meropenem was originally developed by Sumitomo Pharmaceuticals. It is marketed outside Japan by AstraZeneca with the brand names Merrem and Meronem. Other brand names include Zwipen (India, Marketed by Nucleus) Mepem (Taiwan) Meropen (Japan, Korea) and Neopenem (NEOMED India) . It gained FDA approval in July 1996. It penetrates well into many tissues and body fluids including the cerebrospinal fluid, bile, heart valves, lung, and peritoneal fluid.
Meropenem, sold under the brandname Merrem among others, is an intravenous β-lactam antibiotic used to treat a variety of bacterial infections.[1] Some of these include meningitis, intra-abdominal infection, pneumonia, sepsis, and anthrax.[1]
Common side effects include nausea, diarrhea, constipation, headache, rash, and pain at the site of injection.[1] Serious side effects include Clostridium difficile infection, seizures, and allergic reactions including anaphylaxis.[1] Those who are allergic to other β-lactam antibiotics are more likely to be allergic to meropenem as well.[1] Use in pregnancy appears to be safe.[1] It is in the carbapenem family of medications.[1] Meropenem usually results in bacterial death through blocking their ability to make a cell wall.[1] It is more resistant to breakdown by β-lactamase producing bacteria.[1]
Meropenem was patented in 1983.[2] It was approved for medical use in the United States in 1996.[1] It is on the World Health Organization’s List of Essential Medicines.[3] The World Health Organization classifies meropenem as critically important for human medicine.[4]
Medical uses
The spectrum of action includes many Gram-positive and Gram-negative bacteria (including Pseudomonas) and anaerobic bacteria. The overall spectrum is similar to that of imipenem, although meropenem is more active against Enterobacteriaceae and less active against Gram-positive bacteria. It works against extended-spectrum β-lactamases, but may be more susceptible to metallo-β-lactamases.[5] Meropenem is frequently given in the treatment of febrile neutropenia. This condition frequently occurs in patients with hematological malignancies and cancer patients receiving anticancer drugs that suppress bone marrow formation. It is approved for complicated skin and skin structure infections, complicated intra-abdominal infections and bacterial meningitis.
In 2017 the FDA granted approval for the combination of meropenem and vaborbactam to treat adults with complicated urinary tract infections.[6]
Administration
Meropenem is administered intravenously as a white crystalline powder to be dissolved in 5% monobasic potassium phosphate solution. Dosing must be adjusted for altered kidney function and for haemofiltration.[7]
As with other ß-lactams antibiotics, the effectiveness of treatment depends on the amount of time during the dosing interval that the meropenem concentration is above the minimum inhibitory concentration for the bacteria causing the infection.[8] For ß-lactams, including meropenem, prolonged intravenous administration is associated with lower mortality than bolus intravenous infusion in persons with whose infections are severe, or caused by bacteria that are less sensitive to meropenem, such as Pseudomonas aeruginosa.[8][9]
Side effects
The most common adverse effects are diarrhea (4.8%), nausea and vomiting (3.6%), injection-site inflammation (2.4%), headache (2.3%), rash (1.9%) and thrombophlebitis (0.9%).[10] Many of these adverse effects were observed in severely ill individuals already taking many medications including vancomycin.[11][12] Meropenem has a reduced potential for seizures in comparison with imipenem. Several cases of severe hypokalemia have been reported.[13][14] Meropenem, like other carbapenems, is a potent inducer of multidrug resistance in bacteria.
Pharmacology
Mechanism of action
Meropenem is bactericidal except against Listeria monocytogenes, where it is bacteriostatic. It inhibits bacterial cell wall synthesis like other β-lactam antibiotics. In contrast to other beta-lactams, it is highly resistant to degradation by β-lactamases or cephalosporinases. In general, resistance arises due to mutations in penicillin-binding proteins, production of metallo-β-lactamases, or resistance to diffusion across the bacterial outer membrane.[10] Unlike imipenem, it is stable to dehydropeptidase-1, so can be given without cilastatin.
In 2016, a synthetic peptide-conjugated PMO (PPMO) was found to inhibit the expression of New Delhi metallo-beta-lactamase, an enzyme that many drug-resistant bacteria use to destroy carbapenems.[15][16]
Society and culture

Meropenem vial
Trade names
| Country | Name | Maker |
|---|---|---|
| India | Inzapenum | Dream India |
| Aurobindo Pharma | ||
| Penmer | Biocon | |
| Meronir | Nirlife | |
| Merowin | Strides Acrolab | |
| Aktimer | Aktimas Biopharmaceuticals | |
| Neopenem | Neomed | |
| Mexopen | Samarth life sciences | |
| Meropenia | SYZA Health Sciences LLP | |
| Ivpenem | Medicorp Pharmaceuticals | |
| Merofit | ||
| Lykapiper | Lyka Labs | |
| Winmero | Parabolic Drugs | |
| Bangladesh | ||
| Meroject | Eskayef Pharmaceuticals Ltd. | |
| Merocon | Beacon Pharmaceuticals | |
| Indonesia | Merofen | Kalbe |
| Brazil | Zylpen | Aspen Pharma |
| Japan, Korea | Meropen | |
| Australia | Merem | |
| Taiwan | Mepem | |
| Germany | Meronem | |
| Nigeria | Zironem | Lyn-Edge Pharmaceuticals |
| US | Meronem | AstraZeneca |
| … | Merosan | Sanbe Farma |
| Merobat | Interbat | |
| Zwipen | ||
| Carbonem | ||
| Ronem | Opsonin Pharma, BD | |
| Neopenem | ||
| Merocon | Continental | |
| Carnem | Laderly Biotech | |
| Penro | Bosch | |
| Meroza | German Remedies | |
| Merotrol | Lupin) | |
| Meromer | Orchid Chemicals | |
| Mepenox | BioChimico | |
| Meromax | Eurofarma | |
| Ropen | Macter | |
| mirage | adwic | |
| Meropex | Apex Pharma Ltd. | |
| Merostarkyl | Hefny Pharma Group[17] |
References
- ^ Jump up to:a b c d e f g h i j “Meropenem”. The American Society of Health-System Pharmacists. Retrieved 8 December 2017.
- ^ Fischer, Janos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 497. ISBN 9783527607495.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ World Health Organization (2019). Critically important antimicrobials for human medicine (6th revision ed.). Geneva: World Health Organization. hdl:10665/312266. ISBN 9789241515528.
- ^ AHFS Drug Information (2006 ed.). American Society of Health-System Pharmacists. 2006.
- ^ Commissioner, Office of the (24 March 2020). “Press Announcements – FDA approves new antibacterial drug”. http://www.fda.gov.
- ^ Bilgrami, I; Roberts, JA; Wallis, SC; Thomas, J; Davis, J; Fowler, S; Goldrick, PB; Lipman, J (July 2010). “Meropenem dosing in critically ill patients with sepsis receiving high-volume continuous venovenous hemofiltration” (PDF). Antimicrobial Agents and Chemotherapy. 54 (7): 2974–8. doi:10.1128/AAC.01582-09. PMC 2897321. PMID 20479205.
- ^ Jump up to:a b Yu Z, Pang X, Wu X, Shan C, Jiang S (2018). “Clinical outcomes of prolonged infusion (extended infusion or continuous infusion) versus intermittent bolus of meropenem in severe infection: A meta-analysis”. PLOS ONE. 13 (7): e0201667. Bibcode:2018PLoSO..1301667Y. doi:10.1371/journal.pone.0201667. PMC 6066326. PMID 30059536.
- ^ Vardakas KZ, Voulgaris GL, Maliaros A, Samonis G, Falagas ME (January 2018). “Prolonged versus short-term intravenous infusion of antipseudomonal β-lactams for patients with sepsis: a systematic review and meta-analysis of randomised trials”. Lancet Infect Dis. 18 (1): 108–120. doi:10.1016/S1473-3099(17)30615-1. PMID 29102324.
- ^ Jump up to:a b Mosby’s Drug Consult 2006 (16 ed.). Mosby, Inc. 2006.
- ^ Erden, M; Gulcan, E; Bilen, A; Bilen, Y; Uyanik, A; Keles, M (7 March 2013). “Pancytopenýa and Sepsýs due to Meropenem: A Case Report” (PDF). Tropical Journal of Pharmaceutical Research. 12 (1). doi:10.4314/tjpr.v12i1.21.
- ^ “Meropenem side effects – from FDA reports”. eHealthMe.
- ^ Margolin, L (2004). “Impaired rehabilitation secondary to muscle weakness induced by meropenem”. Clinical Drug Investigation. 24(1): 61–2. doi:10.2165/00044011-200424010-00008. PMID 17516692. S2CID 44484294.
- ^ Bharti, R; Gombar, S; Khanna, AK (2010). “Meropenem in critical care – uncovering the truths behind weaning failure”. Journal of Anaesthesiology Clinical Pharmacology. 26 (1): 99–101.
- ^ “New molecule knocks out superbugs’ immunity to antibiotics”. newatlas.com. 20 January 2017. Retrieved 2017-01-25.
- ^ K., Sully, Erin; L., Geller, Bruce; Lixin, Li; M., Moody, Christina; M., Bailey, Stacey; L., Moore, Amy; Michael, Wong; Patrice, Nordmann; M., Daly, Seth (2016). “Peptide-conjugated phosphorodiamidate morpholino oligomer (PPMO) restores carbapenem susceptibility to NDM-1-positive pathogens in vitro and in vivo”. Journal of Antimicrobial Chemotherapy. 72 (3): 782–790. doi:10.1093/jac/dkw476. PMC 5890718. PMID 27999041.
- ^ “Hefny Pharma Group”. hefnypharmagroup.info. Retrieved 2018-05-22.
External links
- “Meropenem”. Drug Information Portal. U.S. National Library of Medicine.
| Clinical data | |
|---|---|
| Trade names | Merrem, others |
| AHFS/Drugs.com | Monograph |
| Pregnancy category | AU: B2 |
| Routes of administration | Intravenous |
| ATC code | J01DH02 (WHO) |
| Legal status | |
| Legal status | AU: S4 (Prescription only)UK: POM (Prescription only)US: ℞-only |
| Pharmacokinetic data | |
| Bioavailability | 100% |
| Protein binding | Approximately 2% |
| Elimination half-life | 1 hour |
| Excretion | Renal |
| Identifiers | |
| showIUPAC name | |
| CAS Number | 119478-56-7 |
| PubChem CID | 441130 |
| DrugBank | DB00760 |
| ChemSpider | 389924 |
| UNII | FV9J3JU8B1 |
| KEGG | D02222 |
| ChEBI | CHEBI:43968 |
| ChEMBL | ChEMBL127 |
| PDB ligand | MEM (PDBe, RCSB PDB) |
| CompTox Dashboard (EPA) | DTXSID7045526 |
| ECHA InfoCard | 100.169.299 |
| Chemical and physical data | |
| Formula | C17H25N3O5S |
| Molar mass | 383.46 g·mol−1 |
| 3D model (JSmol) | Interactive image |
| showSMILES | |
| showInChI | |
| (verify) |
Patent
Publication numberPriority datePublication dateAssigneeTitleUS4888344A *1986-07-301989-12-19Sumitomo Pharmaceuticals Company, LimitedCarbapenem compound in crystalline form, and its production and useCN101348486A *2008-08-292009-01-21深圳市海滨制药有限公司Preparation of meropenemCN101962383A *2010-11-122011-02-02上海巴迪生物医药科技有限公司Synthesis method of meropenemFamily To Family CitationsJPS6475488A *1987-09-171989-03-22Sumitomo PharmaProduction of beta-lactam compound* Cited by examiner, † Cited by third party
Publication numberPriority datePublication dateAssigneeTitleFamily To Family CitationsCN101962383A *2010-11-122011-02-02上海巴迪生物医药科技有限公司Synthesis method of meropenemCN102250096B *2011-09-052016-04-06江西华邦药业有限公司A kind of preparation method of meropenemCN104072523B *2014-07-142017-10-24上海上药新亚药业有限公司The preparation method of BiapenemCN108191869A *2018-01-222018-06-22重庆天地药业有限责任公司The purification process of Meropenem
PublicationPublication DateTitleEP0007973B11984-02-01Process for the preparation of thienamycin and intermediatesUS4631150A1986-12-23Process for the preparation of penemsWO2012062035A12012-05-18Synthesis method for meropenemWO2010022590A12010-03-04Method for preparation of meropenemUS4443373A1984-04-17Process for the production of antibiotic penemsWO2008035153A22008-03-27Process for the preparation of beta-lactam antibioticEP0167154B11990-01-03Process for preparing 4-acetoxy-3-hydroxyethylazetizin-2-one derivativesKR101059339B12011-08-24Method for preparing carbapenem compound for oral administrationKR100886347B12009-03-03Process for stereoselective preparation of 4-BMA using a chiral auxiliaryUS4841043A1989-06-20Stereoselective synthesis of 1-β-alkyl carbapenem antibiotic intermediatesUS4772683A1988-09-20High percentage beta-yield synthesis of carbapenem intermediatesJP2000344774A2000-12-12Production of carbapenem compoundAU745980B22002-04-11Titanium catalyzed preparation of carbapenem intermediatesUS5700930A1997-12-234-substituted azetidinones as precursors to 2-substituted-3-carboxy carbapenem antibiotics and a method of producing themJP2002338572A2002-11-27Method for producing carbapenemsJP3684339B22005-08-17Method for producing carbapenem compoundsEP0066301B11986-01-22Intermediates for the preparation of thienamycin and process for preparing the sameWO2001053305A12001-07-26Processes for the preparation of carbapenem derivativesAU737502B22001-08-23Preparation of beta-methyl carbapenem intermediatesJP3213734B22001-10-02New β-lactam compoundsJP2004107289A2004-04-08Method for producing vinyl sulfide compoundJPH085853B21996-01-24Lactam compound and its manufacturing methodJPH0827168A1996-01-30Carbapenem intermediate fieldEP0204440A11986-12-10Azetidine derivatives productionWO1994021638A11994-09-29Process for the preparation of condensed carbapeneme derivatives
ApplicationPriority dateFiling dateTitleCN 2010105416652010-11-122010-11-12Synthesis method of meropenemCN201010541665.52010-11-12
Nmrhttps://www.researchgate.net/figure/1HNMR-spectra-of-meropenem-hydrolysis-catalyzed-by-NDM-1-Ecoli-cells-Only-1H-signals-of_fig3_272515470


NMRNMR spectra monitoring meropenem hydrolysis catalyzed by NDM-1. a¹H NMR spectrum of hydrolyzed meropenem recorded before and 6 or 20 min after NDM-1 addition to the reaction system. b Part of a ROESY spectrum of the hydrolysis product. Diagonal and cross peaks are shown in blue and red, respectively. Proton signal assignments are labeled beside the peaks. The chemical shifts of H2, H1, H5, and H10 are highlighted by dashed linesSEEhttps://www.mdpi.com/1420-3049/23/11/2738/htm
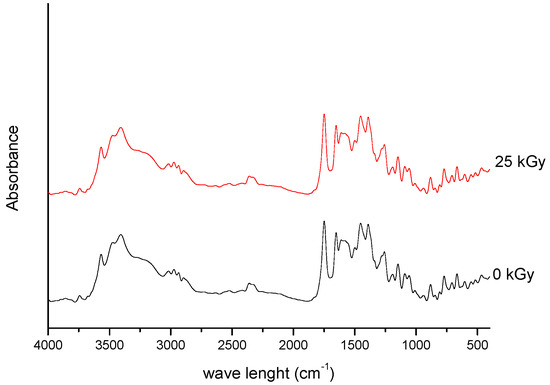
Figure 1. FT-IR spectra of unirradiated and irradiated (25 kGy) meropenem.
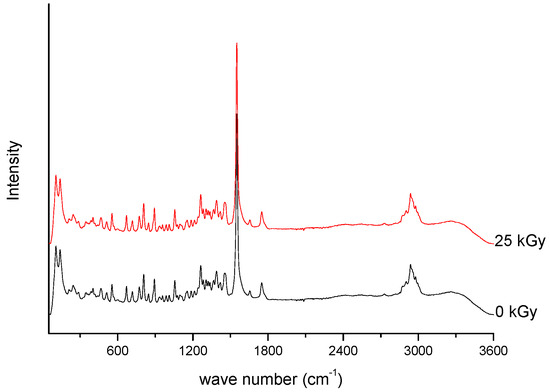
Figure 2. Raman spectra of unirradiated and irradiated (A-25 kGy) meropenem.
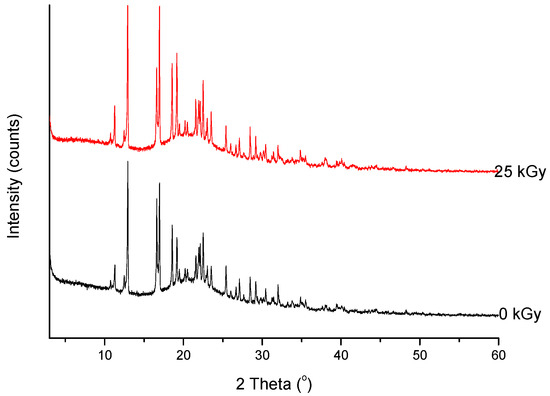
Figure 6. XRPD diffractograms of unirradiated and irradiated (25 kGy) meropenem.
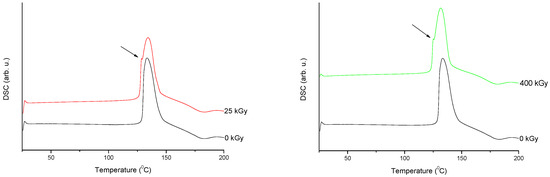
Figure 7. Differential scanning calorimetry (DSC) curves of non-irradiated and irradiated (A-25 kGy, B-400 kGy) meropenem. The arrows indicate the changes in the DSC spectrum after irradiation.
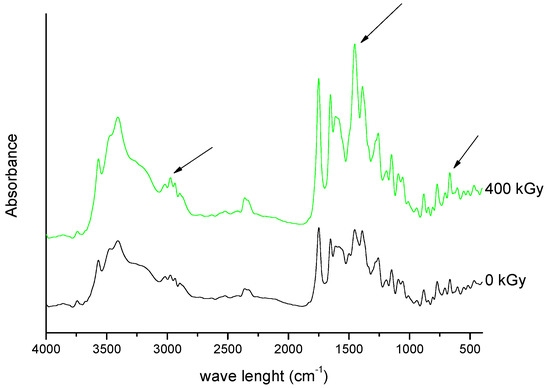
Figure 9. FT-IR spectra of unirradiated and irradiated (400 kGy) meropenem. The arrows indicate the changes in the FT-IR spectrum after irradiation.
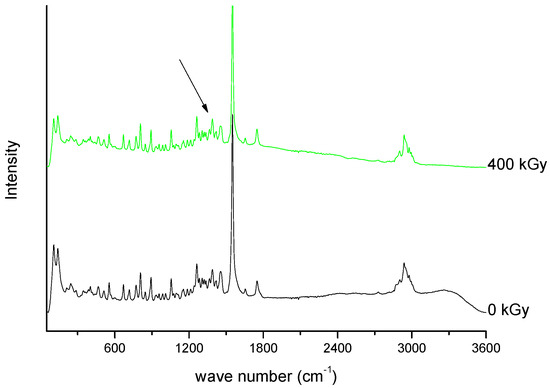
Figure 10. Raman spectra of unirradiated and irradiated (400 kGy) meropenem. The arrow indicates the change in the Raman spectrum after irradiation.
//////////////Meropenem, Merrem, intravenous β-lactam antibiotic, bacterial infections, meningitis, intra-abdominal infection, pneumonia, sepsis, anthrax, Antibiotic SM 7338, ICI 194660, SM 7338, ANTIBACTERIALS
[H][C@]1([C@@H](C)O)C(=O)N2C(C(O)=O)=C(S[C@@H]3CN[C@@H](C3)C(=O)N(C)C)[C@H](C)[C@]12[H]
