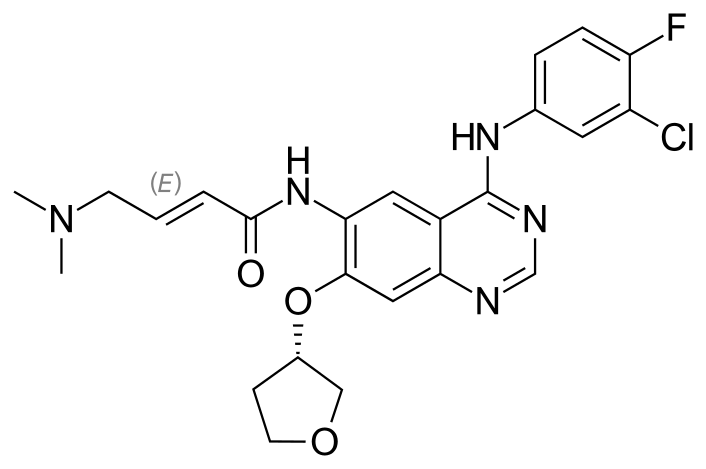
Afatinib
439081-18-2
850140-73-7 dimaleate
Tovok, BIBW2992, Tomtovok
An irreversible EGFR/HER2 inhibitor
| Molecular Weight: | 485.94 |
| Molecular Formula: | C24H25ClFN5O3 |
N-[4-[(3-Chloro-4-fluorophenyl)amino]-7-[[(3S)-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4(dimethylamino)-2-butenamide
4 - [(3-chloro-4-fluorophenyl) amino] -6 – {[4 - (N, N-dimethylamino)-1-oxo-2-buten-1-yl] – amino} -7 – ((S )-tetrahydrofuran-3-yloxy)-quinazoline
(E)-4-Dimethylamino-but-2-enoic acid {4-(3-chloro-4-fluoro- phenylanimo)-7-[(S)-(tetrahydro-furan-3-yl) oxy]-quinazolin-6-yl} -amide
4 – [(3_ chloro-4 - fluorophenyl) amino] -6 – {[4_ (N, N-dimethylamino)-buten-1-oxo-_2_ - yl] amino}-7 – ((S) – tetrahydrofuran-3 – yloxy) – quinazoline
The endorsement for Giotrif (afatinib) covers the drug’s use in the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) who have the epidermal growth factor receptor (EGFR) gene mutation, which is present in about 10 per cent of people with NSCLC.
It caps a good month for Boehringer, which won US approval for the drug under the brand name Gilotrif two weeks ago, adding to the company’s list of therapy areas, which so far include chronic obstructive pulmonary disease (COPD), anticoagulation, HIV, Parkinson’s disease and diabetes.
In the US, the drug is approved alongside a companion diagnostic to help determine if a patient’s lung cancer cells express the EGFR mutations, whereas the EMA recommendation just includes the requirement that Giotrif be initiated and supervised by a physician experienced in the use of anti-cancer therapies.
http://www.pmlive.com/pharma_news/boehringers_first_cancer_drug_leads_ema_recommendations_493051
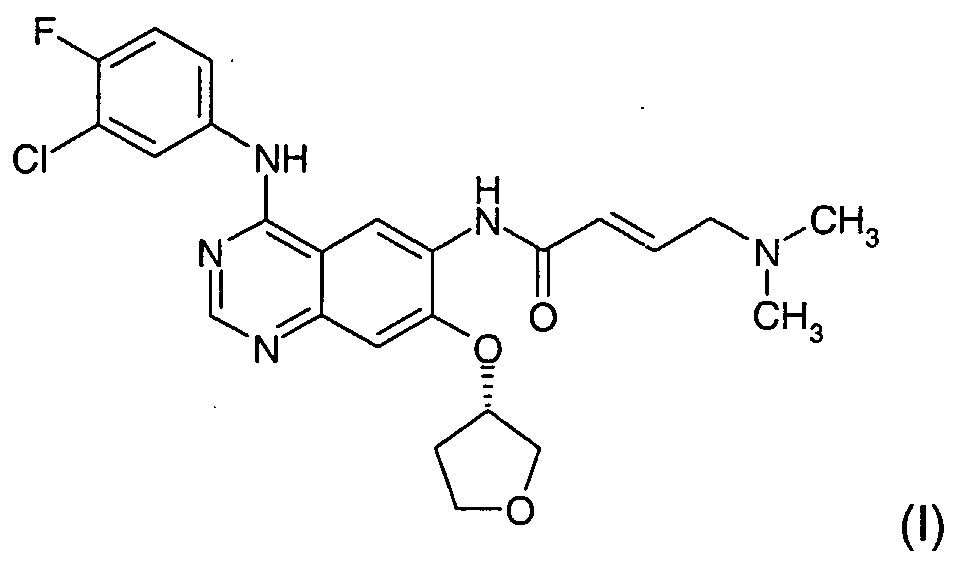
GILOTRIF tablets contain afatinib, a tyrosine kinase inhibitor which is a 4-anilinoquinazoline. Afatinib is presented as the dimaleate salt, with the chemical name 2-butenamide, N-[4-[(3-chloro-4-fluorophenyl)amino]7-[[(3S)-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4-(dimethylamino)-,(2E)-, (2Z)-2-butenedioate (1:2). Its structural formula is:
 |
Afatinib dimaleate is a white to brownish yellow powder, water soluble and hygroscopic, with an empirical formula of C32H33ClFN5O11, and a molecular weight of 718.1 g/mol.
GILOTRIF tablets for oral administration are available in 40 mg, 30 mg, or 20 mg of afatinib (equivalent to 59.12 mg, 44.34 mg, or 29.56 mg afatinib dimaleate, respectively). The inactive ingredients of GILOTRIF are the following: Tablet Core: lactose monohydrate, microcrystalline cellulose, crospovidone, colloidal silicon dioxide, magnesium stearate. Coating: hypromellose, polyethylene glycol, titanium dioxide, talc, polysorbate 80, FD&C Blue No. 2 (40 mg and 30 mg tablets only).
Afatinib (BIBW2992) is an irreversible EGFR/Neu inhibitor with an IC50 of 14 nM. Afatinib is a potent inhibitor of EGFR phosphorylation. Afatinib showed positive results in assays against a variety of human cancer cell lines, including A431, murine NIH-3T3 cells, and breast cancer cell line BT-474.
Afatinib[2] (INN; trade name Gilotrif in the US and Giotrif in Europe, previously Tomtovok and Tovok[3]) is a drug approved inmuch of the world (including the United States, Canada, the United Kingdom and Australia) for the treatment of metastatic non-small cell lung carcinoma (NSCLC), developed by Boehringer Ingelheim.[4][5][6] It acts as an angiokinase inhibitor.
Quinazoline derivatives, such as afatinib, are described in WO2002050043. This document also describes certain favourable pharmacological properties of this compound. The dimaleate salt and its crystalline form are described in WO2005037824.
It is known in the W002/50043, which describes the pharmacological properties has important compounds include in particular their pharmacological properties mediated by the tyrosine kinase inhibitory effect and the signal transmission through the skin growth factor receptor (EGF-R) signal transduction mediated inhibitory effect. Therefore, this type of compounds are useful in the treatment of diseases, in particular for the treatment of tumor diseases, lung and gastrointestinal and respiratory tract and gall bladder and bile duct disease.
W002/50043 discloses a method for preparing a compound wherein the amino crotonic group (IV), such as 4_ [(3 - chloro-4 - fluorophenyl) amino] -6 – {[4 - (N, N-two methyl-amino)-oxo-2-1_ - buten-1 - yl] amino} -7 – ((S) – tetrahydrofuran-3 – yloxy) – quinazoline in the one-pot reaction from the corresponding aniline component (II), bromo crotonic acid (III), oxalyl chloride and a secondary amine prepared (see Figure 1).
Figure 1:
In the method, the yield was 50% at most. In addition, the implementation typically purified by column chromatography. Therefore Preparation of 4 – [(3_ chloro-4 - fluorophenyl) amino] -6 – {[4 - (N, N-dimethylamino)-l-oxo-2 - buten-1 - yl] amino} -7 – ((S) – tetrahydrofuran-3 – yloxy) – quinazoline of the method is not for large-scale industrial production. Moreover, the method is not drawback bromo crotonate purchased by a large number of commercial sources, and the corresponding bromo-methyl crotonate only be obtained in a purity of about 80%.These methods are used in this case is also 4 – [(3 - chloro-4 - fluorophenyl) amino] -6 – {[4 - (N, N-dimethylamino) -1 - oxo - butene-1 - yl] amino} -7 – (⑶ – tetrahydrofuran-3 – yloxy) – quinazoline industrialized production adversely affect the applicability.
In the above-mentioned drawbacks of known production methods, the present invention is to provide a produce aminocrotonate aryl amides, in particular 4 – [(3 - chloro-4 - fluorophenyl) amino] -6 – {[4 - (N, N-dimethylamino)-buten-1-oxo-_2_ - yl] amino} -7 – ((S) – tetrahydrofuran-3 – yloxy) – quinazoline The method of the method can be easily obtained using high purity starting materials and does not require the use of any material technology. Thus, the new method should be applicable on an industrial scale synthesis grade and therefore suitable for commercial applications.
This task is according to the present invention for preparing 4 – [(3 - chloro-4 - fluorophenyl) amino] -6 – {[4 - (N, N-dimethylamino) -1 - oxo-2 - buten-1 - yl] amino} -7 – (⑶ – tetrahydrofuran-3 – yloxy) – quinazoline, and other amino crotonic method based compound. In addition to high yield industrially embodiment, the synthesis method according to the present invention also has a very good purity and less than 0.1 of the advantages of a low cis content.
According to Figure 2, in the method according to the present invention, an aryl group corresponding amino compound (V) with two – (Ch-ware yl) _ phosphono acetic acid, preferably with diethyl phosphonoacetate, by After appropriate activation, in a suitable reaction solvent, preferably for the use of the active 1,1 – carbonyldiimidazole, 1,1 – carbonyldiimidazole – triazole or propane phosphonic acid anhydride, is preferred for the use of 1, 1 – carbonyl diimidazole. The solvent used may be, for example, tetrahydrofuran (THF), dimethylformamide (DMF) or ethyl acetate.
The amide may be connected through any possible approach for activation, i.e., for example, 1,1 _ carbonyldiimidazole, 1,1 – carbonyldiimidazole – triazole, DCC (N, N-dicyclohexyl carbodiimide ), EDC (N ‘_ (dimethylaminopropyl)-N-ethylcarbodiimide), TBTU (0 – (benzotriazol-1 – yl)-N, N, N’, N ‘ – pan tetramethyluronium tetrafluoroborate), thiazolidine-2 – thione, or through the use of thionyl chloride may be converted to the corresponding acyl chloride. If desired, activation may be used an organic base such as triethylamine or pyridine embodiment, and can additionally added DMAP (dimethylaminopyridine). Suitable solvents include DMF, THF, ethyl acetate, toluene, chlorinated hydrocarbons or mixtures thereof.
http://www.google.com/patents/CN1867564B?cl=en
Example 1
[0078] {[4 - (3 - chloro-4 - fluoro - phenylamino) -7 - (⑶ - tetrahydrofuran _3_-yloxy) - quinazoline _6_ yl carbamoyl] methyl}-_ _ Diethyl
[0079]
A 3. 58kg of 1,1 _ carbonyldiimidazole (22.16 mol) was placed in 12.8 l of tetrahydrofuran, and at a temperature of 40 ° C was dissolved in it with 6.5 l of tetrahydrofuran, 4. 52kg (22. 16 mol) of diethyl phosphono acetic acid mixture. Temperature at 40 ° C the mixture was stirred for 30 minutes. The resulting solution was referred to as Solution A.
A 6. 39kg (17. 05 moles) of N4-(3_ _4_ chloro fluoro – phenyl) _7_ (tetrahydrofuran _3_ yloxy) quinazoline-4, 6 – diamine Add 26 5 of tetrahydrofuran at 40 ° C and the solution A were mixed and stirred at a temperature 30 ° C for 2 hours.To the suspension was added 64 l tert-butyl methyl ether and, after cooling to 20 ° C, the precipitate was removed by centrifugation. Using 16 liters of tetrahydrofuran and 16 l of a mixture of tert-butyl methyl ether, washed, and then washed with 32 liters of water and dried at 50 ° C.
[0082] Yield: 6. 58kg (69. 8%) of white crystals, the content = HPLC 99. IFl%
[0083] Example 2
[0084] (E) -4 – dimethylamino – D -2 – acid – [4 - (chloro-3_ _4_ fluoro - phenylamino) _7_ (⑶ - tetrahydrofuran-3 - yloxy) - quinoline yl-6 - yl] – amide
[0085]
[0086] A 5.6 l of 30% hydrochloric acid (53.17 mol) was added to 4.4 liters of water. Then the temperature is under 30 ° C was added dropwise over 20 minutes 4. 28kg 95% of (dimethylamino) _ acetaldehyde – diethyl acetal (26.59 mol).Temperature at 35 ° C the reaction solution was stirred for 8 hours was cooled to 5 ° C and kept under argon. This solution is called Solution B.
[0087] A 4. 55kg (68. 06 mol) of potassium hydroxide dissolved in 23.5 liters of water and cooled to _5 ° C. This solution is called Solution C.
[0088] A 5. 88kg (10. 63 mol) ((4_ (3_ _4_ chloro fluoro – phenylamino) _7_ (tetrahydrofuran _3_-yloxy) – quinazolin-6 – yl carbamoyl) – methyl)-phosphonic acid diethyl ester and 0.45kg _ lithium chloride (10.63 moles) was placed in 23.5 l of tetrahydrofuran and cooled to -7 ° C. Was added over 10 minutes a cold solution of C. Then _7 ° C temperature of the solution was added over 1 hour B. At _5 ° C temperature for 1 hour under stirring the reaction mixture was heated to 20 ° C and mixed with 15 liters of water. After cooling to; TC temperature, the suspension was suction filtered, the precipitate was washed with water and dried. Yield: 5.21kg The crude product, 100%, water content: 6.7%.
[0089] Using Titanium Dioxide / methyl cyclohexane embodiment the crystallization of the crude product.
[0090] Yield: 78%, purity: HPLC99. 4F1%, water content: 5.4%
[0091] Example 3
[0092] (E) -4 – dimethylamino – D -2 – acid – (4 – (chloro-3_ _4_ fluoro – phenylamino) ~ 7 ~ ((S) – tetrahydrofuran-3 – yl oxy) – quinazolin-6 – yl) – amide dimaleate
[0093] A 6. Okg (12. 35 mol) of (E_) _4_ _2_ dimethylamino acid _ D – (4_ (3_ _4_ chloro fluoro – phenylamino) -7 – ((S) – tetrahydrofuran-3 – yloxy) – quinazolin-6 – yl) – amide into 84 liters of ethanol and heated to 70 ° C, and dissolved in 36 l of ethanol and 2.94kg (25.31 moles) of maleic acid was mixed . At the beginning of crystallization, the first mixture was cooled to 20 ° C and stirred for 2 hours and then at 0 ° C temperature for 3 hours. Precipitate was suction filtered, washed with 19 l of ethanol at a temperature of 40 ° C in vacuo.
[0094] Yield: 8. Ilkg (91. 5%)
[0095] Melting point: 178 ° C
[0096] 1H-NMR (CD3OD): δ = 2. 47 + 2. 27 (m + m, 2H), 2. 96 (s, 6H), 4. 03 (m, 2Η), 4. 07 +3 . 92 (m + m, 2Η), 4. 18 +4. 03 (m + m, 2Η), 5. 32 (m, 1Η), 6. 26 (s, 4H), 6. 80 (m, 1H ), 6. 99 (m, 1H), 7 · 27 (s, 1Η), 7 · 30 (t, 1Η), 7 · 66 (m, 1Η), 7 · 96 (dd, 1Η), 8 · 62 (s, 1Η), 9 · 07 (s, 1Η) ppm
13
…………….
Examples:
Example 1
{[4 - (3-chloro-4-fluoro-phenylamino) -7 - ((S)-tetrahydrofuran-3-yloxy)-quinazolin-6-ylcarbamoyl]-methyl)-phosphonic acid diethyl ester
3.58 kg 1 ,1-carbonyldiimidazole (22.16 mole) were placed in 12.8 liters of tetrahydrofuran at 40 ° C with 4.52 kg (22.16 mol) diethylphosphonoacetic acid, dissolved in 6.5 liters of tetrahydrofuran, . The mixture is stirred for 30 minutes at 40 ° C. The solution thus obtained is referred to as solution A.
6.39 kg (17.05 mol) of N 4 - (3-chloro-4-fluoro-phenyl) -7 – (tetrahydrofuran-3-yloxy) quinazolin-4,6-diamine in 26.5 liters of tetrahydrofuran and submitted to 40 ° C and mixed with the solution A and stirred at 30 ° C for 2 hours. To 64 liters of suspension of tert -. Added butyl methyl ether and, after cooling to 20 ° C., the precipitate is removed by centrifugation. It is dried with a mixture of 16 liters and 16 liters of tetrahydrofuran tert-butyl methyl ether and then washed with 32 liters of water at 50 ° C. Yield: 6.58 kg (69.8%) of white crystals Assay: HPLC 99.1 area% Example 2
(E)-4-dimethylamino-but-2-enoic acid [4 – (3-chloro-4-fluoro-phenylamino) -7 – ((S) – tetrahvdrofuran-3-yloxy)-quinazolin-6yl1 amide
5.6 liters to 4.4 liters of water are added 30% hydrochloric acid (53.17 mol). Then 4.28 kg 95% pure (dimethylamino) acetaldehyde diethyl acetal (26.59 mol) at 30 ° C was added dropwise over 20 minutes. The reaction solution is stirred for 8 hours at 35 ° C, cooled to 5 ° C and kept under argon. This solution is referred to as solution B.
4.55 kg (68.06 mol) of potassium hydroxide are dissolved in 23.5 liters of water and cooled to -5 ° C. This solution is called solution C..
5.88 kg (10.63 mol) of ((4 – (3-chloro-4-fluoro-phenylamino) -7 – (tetrahydrofuran-3-yloxy) – quinazolin-6-ylcarbamoyl)-methyl)-phosphonic acid diethyl ester, and 0.45 kg lithium chloride (10.63 mole) were placed in 23.5 liters of tetrahydrofuran and cooled to -7 ° C. The cold solution C is added within 10 minutes. The solution B is added at -7 ° C over 1 hour. After stirring for one hour at -5 ° C, the reaction mixture is heated to 20 ° C and mixed with 15 liters of water. After cooling to 3 ° C, the suspension is filtered with suction, the precipitate washed with water and dried. Yield: 5.21 kg raw 100% Water content: 6.7%
The crystallization of the raw product is butyl acetate / methylcyclohexane yield: 78% HPLC purity 99.4 area%, water content 5.4% Example 3
(E)-4-dimethylamino-but-2-enoic acid (4 – (3-chloro-4-fluoro-pheny hvdrofuran-3-yloxy)-quinazolin-6YL) amide dimaleate
6.0 kg (12.35 mol) of (E)-4-dimethylamino-but-2-enoic acid (4 – (3-chloro-4-fluoro-phenyl-amino) -7 – ((S)-tetrahydrofuran- 3-yloxy) quinazolin-6YL)-amide are in 84 liters
Submitted ethanol and heated to 70 ° C and a solution of 2.94 kg (25.31 mol) of maleic acid in 36 liters of ethanol added.Following the onset of crystallization is first cooled to 20 ° C. and stirred for 2 hours, then 3 hours at 0 ° C. The precipitate is filtered off, washed with 19 liters of ethanol and dried in vacuum at 40 ° C.
Yield: 8.11 kg (91, 5%)
Mp: 178 ° C.
1 H NMR (CD 3 OD): δ = 2.47 + 2.27 (m + m, 2H), 2.96 (s, 6H), 4.03 (m, 2H), 4.07 + 3 , 92
(M + m, 2H), 4.18 + 4.03 (m + m, 2H), 5.32 (m, 1 H), 6.26 (s, 4H), 6.80 (m, 1 H ), 6.99 (m, 1 H), 7.27 (s, 1 H), 7.30 (t, 1 H), 7.66 (m, 1 H), 7.96 (dd, 1 H ), 8.62 (s, 1 H), 9.07 (s, 1H) ppm
…………..

U.S. Patent No. : 8,426,586 patent expires : October 10, 2029
WO200250043A1 (compound);
WO2003094921A2 (anticancer purposes);
WO2003066060A2 (anti-inflammatory purposes);
US2005085495A1 (process);
WO2005037824A2 (process);
WO2007085638A1 (process);
US2011207932A1 (process);
WO2011084796A2 (deuterated);
WO2012121764A1 (crystalline);
WO2013052157A1 (crystalline)
Chinese patents : CN1867564
CN101402631
|
5-30-2012
|
Amide derivative for inhibiting the growth of cancer cells
|
|
|
6-15-2011
|
PROCESS FOR PREPARING AMINOCROTONYLAMINO-SUBSTITUTED QUINAZOLINE DERIVATIVES
|
|
|
12-25-2009
|
METHOD FOR TREATING CANCER HARBORING EGFR MUTATIONS
|
|
|
12-11-2009
|
QUINAZOLINE DERIVATIVES FOR THE TREATMENT OF CANCER DISEASES
|
|
|
12-11-2009
|
COMBINATION TREATMENT OF CANCER COMPRISING EGFR/HER2 INHIBITORS
|
|
|
9-12-2008
|
Multi-Functional Small Molecules as Anti-Proliferative Agents
|
|
|
4-22-2005
|
Process for preparing amino crotonyl compounds
|
Filed under: cancer, Uncategorized Tagged: Afatinib






















































 THE ESTER OF EVACETRAPIB
THE ESTER OF EVACETRAPIB






















 now taken over by sun
now taken over by sun
















































 DR ANTHONY
DR ANTHONY
















