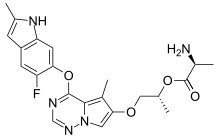
 BMS-582664, brivanib alaninate
BMS-582664, brivanib alaninate
((S)-((R)-1-(4-(4-Fluoro-2-methyl-1H-indol-5-yloxy)-5-
methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy)propan-2-yl) 2-aminopropanoate
Brivanib alaninate is a new oncology therapy with potential applications against a wide variety of tumor types and several stages of disease progression
A prodrug of BMS-540215.
- BMS-540215
- BMS540215
- Brivanib
- UNII-DDU33B674I
BMS 540215, 649735-46-6
| (S)-(R)-1-((4-((4-fluoro-2-methyl-1H-indol-5-yl)oxy)-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yl)oxy)propan-2-yl 2-aminopropanoate | |
| Clinical data | |
|---|---|
| Legal status |
|
| Routes | Oral |
| Identifiers | |
| CAS number | 649735-63-7 |
| ATC code | None |
| PubChem | CID 11154925 |
| ChemSpider | 9330033 |
| ChEMBL | CHEMBL270995 |
| Chemical data | |
| Formula | C22H24FN5O4 |
| Mol. mass | 441.5 g/mol |

C22H24FN5O4 : 441.46
[649735-63-7]
Brivanib alaninate (INN/USAN) also known as BMS-582664 is an investigational, anti-tumorigenic drug for oral administration. The drug is being developed by Bristol-Myers Squibb for the treatment of hepatocellular carcinoma or HCC (also called malignant hepatoma), the most common type of liver cancer. Hepatocellular carcinoma [1] is a primary cancer of the liver and is more common in men than in women. The disease occurs mostly in people who have scarring of the liver (cirrhosis) or after infection with hepatitis B or hepatitis C. Symptoms include pain and swelling in the abdomen, weight loss, weakness, loss of appetite and nausea. Hepatocellular carcinoma is a severe and life-threatening disease that is associated with poor overall survival. [2] While the choice of treatment depends mainly on how advanced the disease is, the only proven therapies to cure the cancer is surgery to remove the tumor and liver transplantation, but these therapies can only be carried out in very few patients. Other treatments includechemotherapy and immunotherapy. Radiofrequency ablation and ethanol injection are also used to remove small tumors.[3]
As a result of poor liver function, metastases, or both, only 10% to 20% of patients undergo surgery. In patients having surgery, the 5-year survival rate is only 25% to 50%. Several chemotherapeutic agents have been evaluated for the treatment of hepatocellular carcinoma. Doxorubicin (trade name Adriamycin; also known as hydroxydaunorubicin), the most widely used agent in HCC, has shown a 4% to 10.5% response rate in patients with HCC.
Studies have shown that the overall response (OR) rate, but not overall survival (OS), doubles when doxorubicin was given in combination with cisplatin, IFN, and 5-fluorouracil. The multitargeted tyrosine kinase inhibitor sorafenib (trade name Nexavar), which inhibits vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor, raf, c-kit, and flt-3, has been shown to inhibit HCC-induced proliferation and angiogenesis.
Sorafenib has also been shown to provide a significant improvement in OS in patients with HCC. Based on these results, researchers concluded that this class of agents may be effective in the treatment of HCC. Brivanib alaninate also inhibits VEGFR and fibroblast growth factorreceptors (FGFR), which is known to play a major role in the etiopathogenesis of HCC. To date, brivanib alaninate has been investigated in 29 studies, including more than 4,000 patients around the world.

This manuscript describes the control strategy for the commercial process to manufacture brivanib alaninate. The active pharmaceutical ingredient is a prodrug which is susceptible to hydrolysis. In addition to controlling hydrolysis, a robust strategy was required in order to control input and process-related impurities. Three significant aspects of control include understanding of the reaction parameters in order to minimize the regioisomer during the alkylation with (R)-propylene oxide, development of a design space through statistical models to control impurity formation, and the use of in situ FT-IR to monitor the hydrogenolysis of the Cbz protecting group.
(S)-((R)-1-(4-(4-Fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[1,2-f][1,2,4]triazin-6-yloxy)propan-2-yl)-2-aminopropanonate (1)

1H NMR (400 MHz, CDCl3) 8.31 (1 H, s), 7.83 (1 H, s), 7.25 (1 H, s), 7.00 (1 H, d, J= 8.6 Hz), 6.95 (1 H, dd, J = 15.4, 8.6 Hz), 6.28 (1 H, s), 5.36–5.30 (1 H, m), 4.08–4.00 (2 H, m), 3.57 (1 H, dd, J = 14.0, 6.9 Hz), 2.47 (3 H, s), 2.40 (3 H, s), 1.66 (3 H, s), 1.38 (3 H, d, J = 6.4 Hz), 1.35 (3 H, d, J = 7.1 Hz).
(S)-((R)-1-(4-(4-Fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[1,2-f][1,2,4]triazin-6-yloxy)propan-2-yl)-2-(benzyloxycarbonylamino)propanonate

1H NMR (400 MHz, CDCl3) 8.17 (1 H, br s), 7.84 (1 H, s), 7.41 (1 H, s), 7.35–7.28 (5 H, m), 7.03 (1 H, d, J = 8.6 Hz), 6.95 (1 H, t, J = 7.7 Hz), 6.30 (1 H, s), 5.36–5.32 (2 H, m), 5.11 (2 H, br s), 4.43–4.40 (1 H, m), 4.02–3.99 (2 H, m), 2.46 (3 H, s), 2.41 (3 H, s), 1.44 (3 H, d, J = 7.2 Hz), 1.38 (3 H, d, J = 7.2 Hz).
Ongoing clinical development program
To further investigate the benefits of brivanib in patients with advanced HCC, a broad-spectrum, global, phase III clinical development plan called the Brivanib studies in HCC patients at RISK (BRISK), has been initiated. Clinical benefits seen with brivanib in the first-line setting, and following the failure of sorafenib therapy, highlight the potential to improve the clinical course of patients with advanced HCC. Brivanib may provide a novel therapeutic option to a growing number of patients for whom no other treatment choice exists.
Regulatory status
On 27 October 2011, orphan designation (EU/3/11/918) was granted by the European Commission to Bristol-Myers Squibb for brivanib alaninate for the treatment of hepatocellular carcinoma.[11] Designated orphan medicinal products are products that are still under investigation and are considered for orphan designation on the basis of potential activity. An orphan designation is not a marketing authorization. As a consequence, demonstration of quality, safety and efficacy is necessary before a product can be granted a marketing authorization. At the time of the orphan designation, several medicines were authorized in the EU for the treatment of hepatocellular carcinoma.
Submission and application
At the time of submission of the application for orphan designation, clinical trials with brivanib alaninate in patients with hepatocellular carcinoma were ongoing. As part of the submission process, Bristol-Myers Squibb has provided sufficient information to show that brivanib alaninate might be of significant benefit for patients with hepatocellular carcinoma because it could provide an alternative for patients who cannot take or for whom existing treatments do not work. Early studies show that it might improve the treatment of patients with this condition, particularly if used when existing treatment had failed. However, this assumption needs to be confirmed at the time of EU marketing authorization, in order to maintain the orphan status.
Synthesis of Brivanib
Route 1
[(1R), 2S]-2-Aminopropionic acid 2-[4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy]-1-methylethyl ester, has the structure of formula I:
and is referred to herein as “Compound I”. Compound I, compositions comprising Compound I, and methods of using Compound I are disclosed in U.S. Pat. No. 6,869,952 B2, which is assigned to the present assignee and is incorporated herein by reference in its entirety.Compound I, a prodrug, is suitable for inhibiting tyrosine kinase activity of growth factor receptors such as VEGFR-2 and FGFR-1 and is useful in the treatment of cancer. Compound I is also useful in the treatment of diseases, other than cancer, which are associated with signal transduction pathways operating through growth factors and anti-angiogenesis receptors such as VEGFR-2.
Typically, in the preparation of a pharmaceutical composition, a form of the active ingredient having desired properties such as dissolution rate, solubility, bioavailability, and/or storage stability is sought. For example, a form of the active ingredient, which has the desired solubility and bioavailability, has sufficient stability that it does not convert during manufacture or storage of the pharmaceutical composition to a different form having different solubility and/or bioavailibility. A form of Compound I is desired having properties and stability that allow the preparation of pharmaceutical compositions suitable for the treatment of diseases such as cancer.
…………………………………..
http://www.google.com/patents/US6869952
EXAMPLE 81
[(1R), 2S]-2-Aminopropionic acid 2-[4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy]-1-methylethyl ester
Step A
A mixture of Example 15 (60 mg, 0.0.16 mmol), N-Cbz-L-alanine (89 mg, 0.4 mmol), HATU (253 mg, 0.4 mmol), DIPEA (103 mg, 0.8 mmol), and DMAP (5 mg) in DMF (1 mL) was stirred overnight. The volatiles were removed in vacuo, and the residue was purified by preparative HPLC to afford homochiral 2-benzyloxyearbonylamino-propionic acid [2-[4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy]]-l-methylethyl ester as a white solid (77 mg, 84% yield).
Step B
A mixture of the compound from step A above (60 mg, 0.11 mmol), Pd/C (6 mg), and ammonium formate (200 mg) in DMF (1.5 mL) were stirred at RT for 30 min. The mixture was diluted with ethyl acetate, and then filtered through a pad of Celite®. The filtrate was washed with water, dried over Na2SO4, and concentrated. The product was mixed with 1 N aqueous HCl and lyophilized to afford the title compound as a white solid (53 mg, 99% yield). MS: (M+H)+=442. 1HNMR (CD3OD): δ 1.45 (d, 3H, J=6.60 Hz), 1.56 (d, 3H, J=7.47 Hz), 2.44 (s, 3H), 2.46 (s, 3H), 4.13 (q, 1H), 4.18 (d, 2H, J=3.96 Hz), 5.45 (m 1H); 6.23 (s, 1H); 6.90 (dd, 1H); 7.10 (d, 1H); 7.66 (s, 1H), 7.75 (s, 1H).
…………………………
Discovery of brivanib alaninate ((S)-((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy)propan-2-yl)2-aminopropanoate), a novel prodrug of dual vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1 kinase inhibitor (BMS-540215)
J Med Chem 2008, 51(6): 1976
http://pubs.acs.org/doi/abs/10.1021/jm7013309

A series of amino acid ester prodrugs of the dual VEGFR-2/FGFR-1 kinase inhibitor 1 (BMS-540215) was prepared in an effort to improve the aqueous solubility and oral bioavailability of the parent compound. These prodrugs were evaluated for their ability to liberate parent drug1 in in vitro and in vivo systems. The l-alanine prodrug 8 (also known as brivanib alaninate/BMS-582664) was selected as a development candidate and is presently in phase II clinical trials.
(R)-1-(4-(4-Fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy)propan-2-ol (1)
(S)-((R)-1-(4-(4-Fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-
yloxy)propan-2-yl) 2-aminopropanoate (8)
1H NMR (500 MHz, CD3OD): 7.75 (s, 1H), 7.66 (s, 1H), 7.10 (d, 1H, J= 10.95 Hz), 6.90 (t, 1H,
J=9.60 Hz), 6.23 (s, 1H), 5.45 (m 1H), 4.18 (d, 2H, J= 3.96 Hz), 4.13 (q, 1H), 2.46 (s, 3H), 2.44 (s,3H), 1.56 (d, 3H, J=7.47 Hz), 1.45 (d, 3H, J=6.60 Hz). LC/MS(ESI+) m/z 442.1 (M+H)+.
M.p. 136-142 oC. Elemental analysis: (C22H24FN5O4:1H2O:1.09HCl): Calc’d: C, 52.95; H, 5.47; N,14.03; F, 3.81; Cl, 7.74. Found: C, 53.16; H, 5.35; N, 14.07; F, 3.72; Cl, 7.74HRMS (calc’d for C22H24FN5O4 M+H+): 442.1891, found: 442.1897.
……………………..
Discovery and preclinical studies of (R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy)propan-2-ol (BMS-540215), an in vivo active potent VEGFR-2 inhibitor
J Med Chem 2006, 49(7): 2143
http://pubs.acs.org/doi/abs/10.1021/jm051106d

A series of substituted 4-(4-fluoro-1H-indol-5-yloxy)pyrrolo[2,1-f][1,2,4]triazine-based inhibitors of vascular endothelial growth factor receptor-2 kinase is reported. Structure−activity relationship studies revealed that a methyl group at the 5-position and a substituted alkoxy group at the 6-position of the pyrrolo[2,1-f][1,2,4]triazine core gave potent compounds. Biochemical potency, kinase selectivity, and pharmacokinetics of the series were optimized and in vitro safety liabilities were minimized to afford BMS-540215 (12), which demonstrated robust preclinical in vivo activity in human tumor xenograft models. The l-alanine prodrug of12, BMS-582664 (21), is currently under evaluation in clinical trials for the treatment of solid tumors.
Preparation of (R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy)propan-2-ol (12).
A mixture of 7 (650 mg, 2.08 mmol), (R)-(+)-propylene oxide (595 mg, 10.4 mmol), and
triethylamine (30 µl) in ethanol (8 mL) was heated at 70 °C in a sealed tube. After 2 h, the solvent was removed in vacuo and the product was purified by flash column chromatography (silica gel, 20% EtOAc/ CH2Cl2) to afford a solid, which was triturated with 50% Et2O in CH2Cl2 to give 12 (410 mg,53% yield) as an off-white solid. 1H NMR (500 MHz, CDCl3) δ 7.84 (s, 1H), 7.41 (s, 1H), 7.11 (d, 1H,J = 11 Hz), 7.02 (t, 1H, J = 8.8 Hz), 6.39 (s, 1H), 4.20-4.30 (m, 1H), 3.8-4.00 (m, 2H), 2.51 (s, 3H),2.45 (s, 3H), 1.31 (d, 3H, J = 8.2 Hz). 13C NMR (125 MHz, DMSO-d6) δ 8.36, 13.3, 20.0, 64.5, 76.36,95.1, 100.0, 105.75, 106.66, 110.17, 115.47, 117.66, 117.8, 129.83, 136.34, 137.64, 144.13, 144.6,146.53, 148.15, 160.71. LC/MS (ESI) m/z 371 ((M+H)+. HPLC Method / tR / purity: method A/ 3.95min/ 99%. HRMS for C19H20FN4O3, calcd: 371.1519, found: 371.1522. Anal. (Calcd. ForC19H19FN4O3): theoretical %C 61.61, %H 5.17, %N 15.13, %F 5.13; found %C 61.35, %H 5.06, %N 14.99, %F 4.88.
…….
References
- National Cancer Institute Dictionary of Cancer Terms
- National Cancer Institute Adult Primary Liver Cancer Treatment (PDQ®)
- National Cancer Institute Adult Primary Liver Cancer Treatment (PDQ®)/Treatment Option Overview
- Huynh, H.; Ngo, V. C.; Fargnoli, J.; Ayers, M.; Soo, K. C.; Koong, H. N.; Thng, C. H.; Ong, H. S. et al. (2008). “Brivanib Alaninate, a Dual Inhibitor of Vascular Endothelial Growth Factor Receptor and Fibroblast Growth Factor Receptor Tyrosine Kinases, Induces Growth Inhibition in Mouse Models of Human Hepatocellular Carcinoma”. Clinical Cancer Research 14 (19): 6146–53. doi:10.1158/1078-0432.CCR-08-0509. PMID 18829493.
- Cai, Zhen-wei; Zhang, Yongzheng; Borzilleri, Robert M.; Qian, Ligang; Barbosa, Stephanie; Wei, Donna; Zheng, Xiaoping; Wu, Lawrence et al. (2008). “Discovery of Brivanib Alaninate ((S)-((R)-1-(4-(4-Fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy)propan-2-yl)2-aminopropanoate), A Novel Prodrug of Dual Vascular Endothelial Growth Factor Receptor-2 and Fibroblast Growth Factor Receptor-1 Kinase Inhibitor (BMS-540215)”. Journal of Medicinal Chemistry 51 (6): 1976–80. doi:10.1021/jm7013309. PMID 18288793.
- Ayers, M.; Fargnoli, J.; Lewin, A.; Wu, Q.; Platero, J. S. (2007). “Discovery and Validation of Biomarkers that Respond to Treatment with Brivanib Alaninate, a Small-Molecule VEGFR-2/FGFR-1 Antagonist”. Cancer Research 67 (14): 6899–906. doi:10.1158/0008-5472.CAN-06-4555. PMID 17638901.
- Bhide, Rajeev S.; Cai, Zhen-Wei; Zhang, Yong-Zheng; Qian, Ligang; Wei, Donna; Barbosa, Stephanie; Lombardo, Louis J.; Borzilleri, Robert M. et al. (2006). “Discovery and Preclinical Studies of (R)-1-(4-(4-Fluoro-2-methyl-1H-indol-5-yloxy)-5- methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy)propan- 2-ol (BMS-540215), an in Vivo Active Potent VEGFR-2 Inhibitor”. Journal of Medicinal Chemistry 49 (7): 2143–6. doi:10.1021/jm051106d. PMID 16570908.
- ClinicalTrials.gov NCT00640471 Cetuximab With or Without Brivanib in Treating Patients With K-Ras Wild Type Tumours and Metastatic Colorectal Cancer
- Allen, E.; Walters, I. B.; Hanahan, D. (2011). “Brivanib, a Dual FGF/VEGF Inhibitor, is Active Both First and Second Line against Mouse Pancreatic Neuroendocrine Tumors Developing Adaptive/Evasive Resistance to VEGF Inhibition”. Clinical Cancer Research 17 (16): 5299–310. doi:10.1158/1078-0432.CCR-10-2847. PMC 3156934. PMID 21622725.
- Finn, R. S.; Kang, Y.-K.; Mulcahy, M.; Polite, B. N.; Lim, H. Y.; Walters, I.; Baudelet, C.; Manekas, D.; Park, J.-W. (2012). “Phase II, Open-label Study of Brivanib as Second-line Therapy in Patients with Advanced Hepatocellular Carcinoma”. Clinical Cancer Research 18 (7): 2090–8. doi:10.1158/1078-0432.CCR-11-1991. PMID 22238246.
- orphan designation
External links
- [1] Brivanib (BMS-582664) in advanced solid tumors (AST): Results of a phase II randomized discontinuation trial (RDT).
- ClinicalTrials.gov NCT00798252 Ascending Multiple-Dose Study of Brivanib Alaninate in Combination With Chemotherapeutic Agents in Subjects With Advanced Cancers
- ClinicalTrials.gov NCT00437437 A Phase I Study to Determine the Effect of Food on Brivanib (BMS-582664)
- ClinicalTrials.gov NCT00633789 Phase II Study of Brivanib (BMS-582664) to Treat Multiple Tumor Types
- ClinicalTrials.gov NCT00888173 Brivanib Alaninate in Treating Patients With Recurrent or Persistent Endometrial Cancer
- ClinicalTrials.gov NCT01253668 Brivanib Metastatic Renal Cell Carcinoma
- [2] Public summary of opinion on orphan designation. Brivanib alaninate for the treatment of hepatocellular carcinoma
- [3] New drug information/Abbreviated Scientific Narrative
| US6869952 * | Jul 18, 2003 | Mar 22, 2005 | Bristol Myers Squibb Company | Such as 4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methyl-pyrrolo(2,1-f)(1,2,4)triazin-6-ol; for treament of cancer |
| US6982265 | May 18, 2000 | Jan 3, 2006 | Bristol Myers Squibb Company | Pyrrolotriazine inhibitors of kinases |
| US7671199 * | Apr 20, 2007 | Mar 2, 2010 | Britsol-Myers Squibb Company | dual inhibitor of VEGFR and FGFR tyrosine kinases; cancer |
| WO2006030941A1 | Sep 13, 2005 | Mar 23, 2006 | Eisai Co Ltd | Simultaneous use of sulfonamide-containing compound and angiogenesis inhibitor |
| WO2006124689A2 | May 12, 2006 | Nov 23, 2006 | Squibb Bristol Myers Co | Combination therapy |
| Reference | ||
|---|---|---|
| 1 | Bennett, J.C. et al., eds., Cecil Textbook of Medicine, 20th Edition, vol. 1, W.B. Saunders Company, publ., pp. 1004-1010 (1996). | |
| 2 | Fabbro, D. et al., “Protein kinases as targets for anticancer agents: from inhibitors to useful drugs“, Pharmacology & Therapeutics, vol. 93, pp. 79-98 (2002). | |
| 3 | Gautschi, O. et al., “Aurora Kinases as Anticancer Drug Targets“, Clin. Cancer Res., vol. 14, No. 6, pp. 1639-1648 (2008). | |
| 4 | Huynh, H. et al., “Brivanib Alaninate, a Dual Inhibitor of Vascular Endothelial Growth Factor Receptor and Fibroblast Growth Factor Receptor Tyrosine Kinases, Induces Growth Inhibition in Mouse Models of Human Hepatocellular Carcinoma“, Clin. Cancer Res., vol. 14, No. 19, pp. 6146-6153 (2008). | |
| 5 | Mass, R.D. , “The HER Receptor Family: a Rich Target for Therapeutic Development“, Int, J. Radiation Oncology Biol. Phys., vol. 58, No. 3, pp. 932-940 (2004). | |
| 6 | Mountzios, G. et al., “Aurora kinases as targets for cancer therapy“, Cancer Treatment Reviews, vol. 34, pp. 175-182 (2008). | |
| 7 | National Cancer Institute, http://www.cancer.gov, Brivanib Active Trial Listing (ID#: 5552473) (Dec. 15, 2008). | |
hplc
HPLC methods
Method A :A linear gradient program using 10% methanol, 90% water, 0.2% H3PO4 (solvent A) and
90% methanol, 10% water, 0.2% H3PO4 (solvent B); t = 0 min, 0% B, t = 4 min, 100% B was
employed on a YMC S5 Combiscreen 4.6 × 50 mm column. Flow rate was 4 mL/min and UV detection
was set to 220 nm. The LC column was maintained at ambient temperature.
Method B: A linear gradient program using 10% methanol, 90% water, 0.2% H3PO4 (solvent A) and
90% methanol, 10% water, 0.2% H3PO4 (solvent B); t = 0 min, 0% B, t = 4 min, 100% B was
employed on a YMC ODS 4.6 x 50 mm column. Flow rate was 4 mL/min and UV detection was set to
220 nm. The LC column was maintained at ambient temperature.
Method C: A linear gradient program using 10% methanol, 90% water, 0.1% trifluoroacetic acid (TFA)
(solvent A) and 90% methanol, 10% water, 0.1% TFA (solvent B); t = 0 min, 0% B, t = 4 min, 100% B
was employed on a Chromolith SpeedROD, 4.6 × 50 mm column. Flow rate was 4 mL/min and UV
detection was set to 254 nm. The LC column was maintained at ambient temperature.
Method D: A linear gradient program using 10% methanol, 90% water, 0.1% TFA (solvent A) and 90%
methanol, 10% water, 0.1% TFA (solvent B); t = 0 min, 0% B, t = 2 min, 100% B was employed on a
Waters Xterra 5 m, 4.6 mm × 30 mm column. Flow rate was 4 mL/min and UV detection was set to
220 nm. The LC column was maintained at ambient temperature.
http://www.google.com/patents/EP2364699A1?cl=en
- (R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[1,2-f][1,2,4]triazin-6-yloxy)propan-2-ol (hereinafter also referred to as “BMS-540215″; Proceedings of the American Association for Cancer Research., 46, (Abstract 3033), 2005) (see Formula (XXXIII)):
and
- (28) (S)-((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[1,2-f][1,2,4]triazin-6-yloxy)propan-2-ol) 2-aminopropanonate (hereinafter also referred to as “BMS-582664″; Proceedings of the American Association for Cancer Research., 46, (Abstract 3033), 2005) (see Formula (XXXIV)):
Filed under: cancer Tagged: BMS-582664, Brivanib alaninate






