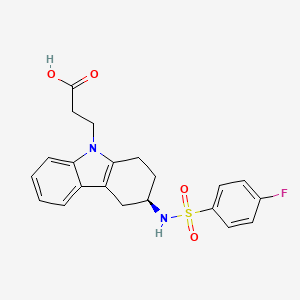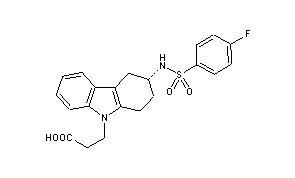
- 3-(4-fluorophenylsulfonamido)-1,2,3,4-tetrahydro-9-carbazole propanoic acid
- BAY u 3405
- BAY u 3406
- BAY u-3405
- BAY u3405
- ramatroban
Ramatroban (INN) is a thromboxane receptor antagonist.[1]
It is also a CRTH2 antagonist.[2]
It is indicated for the treatment of coronary artery disease.[3] It has also been used for the treatment of asthma.[4]
It was developed by the German pharmaceutical company Bayer AG and is co-marketed in Japan by Bayer and Nippon Shinyaku Co. Ltd. under the tradename Baynas.
…………………

The synthesis of Bay u 3405 was carried out as follows: Reductive amination of 3-oxo-1,2,3,4-tetrahydrocarbazole (I) with S-phenethylamine (II) afforded a mixture of diastereomeric amines, of which the desired isomer (III) crystallized in high diastereomeric purity as the hydrogensulfate. Cleavage of the phenethyl group by transfer hydrogenolysis with amminium formate and palladium on charcoal yielded the enantiomerically pure (3R)-3-amino-1,2,3,4-tetrahydrocarbazole (IV). Sulfonylation of (IV) with 4-fluorobenzenesulfonyl chloride (V) to the sulfonamide (VI) followed by addition of acrylonitrile and subsequent hydrolysis gave Bay u 3405.
……………..

The synthesis of [14C]-labeled Bay-u-3405 by two closely related ways has been described: 1) [14C]-Labeled aniline (I) is diazotized and reduced with sodium sulfite, yielding the labeled hydrazine (II), which is condensed with the monoketal of cyclohexane-1,4-dione (III) under Fisher’s indole synthesis (ZnCl2) to afford the tetrahydrocarbazole (IV). The hydrolysis of (IV) with HCl in THF/water yields 1,2,3,4-tetrahydrocarbazol-3-one (V), which is submitted to a reductive condensation with (S)-1-phenylethylamine (VI) by means of tetrabutylammonium borohydride, yielding preferentially the secondary amine (VII), which, after purification, is dealkylated with ammonium formate and Pd/C to afford 1,2,3,4-tetrahydrocarbazole-3(R)-amine (VIII). The acylation of (VIII) with 4-fluorophenylsulfonyl chloride (IX) gives the corresponding sulfonamide (X), which is condensed with acrylonitrile by means of NaH, yielding 3-[3(R)-(4-fluorophenylsulfonamido)-1,2,3,4-tetrahydrocarbazol-9-yl]pro pionitrile (XI). Finally, this compound is hydrolyzed in the usual way. 2) The condensation of the sulfonamide (X) with methyl acrylate by means of NaH as before gives 3-[3(R)-(4-fluorophenylsulfonamido)-1,2,3,4-tetrahydrocarbazol-9-yl]propionic acid methyl ester (XII), which is finally hydrolyzed in the usual way.
……………………
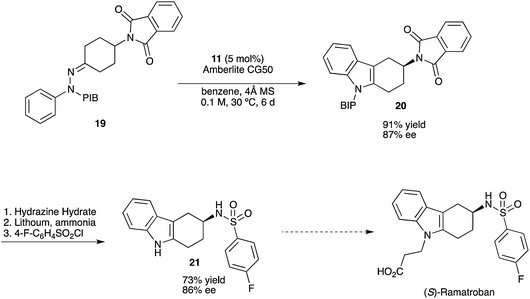
http://pubs.rsc.org/en/content/articlelanding/2012/oc/c2oc90018a#!divAbstract
………………….

http://onlinelibrary.wiley.com/doi/10.1002/adsc.201300993/abstract
This invention relates to 2-amino- tetrahydrocarbazole-propanoic acid and a new process for its synthesis .
2-Amino-tetrahydrocarbazole-propanoic acid is a key intermediate for the synthesis of Ramatroban, a thromboxaneA2 receptor (TP) antagonist with clinical efficacy in asthma and allergic rhinitis.
Ramatroban l-Amino-tetrahydrocarbazole-proanoic acid
US Patent 4988820 discloses the synthesis of this compound stating from compound 1, which is condensed with phenylhydrazine and ring-closed to give indole 2. Deprotection of 2 using acid provides ketone 3. Reductive amination of ketone with s-phenylethylamine in the presence of tetrabutylammonium borohydride provides compound 4, which undergoes palladium catalyzed hydrogenation to give key intermediate 5.
Ramatroban
The process, however, has disadvantages: the starting material 1 is relatively expensive, and the yield of the amination step is only 40% and needs expensive tetrabutylammonium borohydride as the reducing agent. And also the subsequent hydrogenation provides only 70% of the desired compound 5. [0006] US Patent 4988820 also describes an alternative synthesis of compound 5 starting from compound 6, which is oxidized by chromium trioxide to afford ketone 7. Condensation of compound 7 with phenylhydrazine and ring closure give indole 8. The subsequent hydrolysis using HCl provides indole 9. The intermediate 5 is obtained by resolution of racemic 9 using ( + ) -mandelic acid as the resolving agent.
9 5
However, this process has crucial disadvantages: the first step oxidation reaction needs the heavy metal reagent chromium trioxide, which is toxic and expensive, and the resolution of indole 9 using (+) -mandelic acid affords only -10 % of compound 5.
US Patent 5684158 discloses the synthesis of 2- amino-tetrahydrocarbazole-propanoic acid ethyl ester 10 by the alkylation of compound 5 in the presence of about 1 mol of alkali metal hydroxides and phase-transfer catalysts such as potassium hydroxide and benzyltriethylammonium chloride.
The problem with this reaction is that the insoluble material in the reaction mixture becomes very sticky during the reaction. The reaction mixture must be filtered in hot solvent in order to remove insoluble material during work up and the sticky material tents to block the filtration. [0010] Therefore, there is a great need for a new process for the synthesis of 2-amino-tetrahydrocarbazole- propanoic acid.
-
0.91 g 9-(2-Cyanoethyl)-4-[N-(4-fluorphenylsulfonyl)-N-(2-cyanoethyl)aminomethyl]-1,2,3,4-tetrahydrocarbazol be hydrolyzed analogously to Example 7. One obtains 0.77 g (89% of theory) of crystalline product as the sodium salt.
-
M.p .: 160 ° CR f = 0.57 CH 2 Cl 2: CH 3 0H = 9: 1
-
5.8 g (0.0128 mol) of Example 67 are dissolved in 60 ml isopropanol, treated with 130 ml of 10% potassium hydroxide solution, after 16 hours heating under reflux, is cooled, diluted with water and extracted with ethyl acetate. The aqueous phase is concentrated in vacuo and then treated dropwise with vigorous stirring with conc.Hydrochloric acid. The case precipitated acid is filtered off, washed with water and dried thoroughly in vacuo.Obtained 4.4 g (86.6% of theory) of the product. .: Mp 85-95 ° C rotation [α] 20 = 42.55 ° (CHCl 3) D
Example 70
-
The preparation of Example 70 from Example 68 is carried out analogously to the preparation of Example 69 from Example 67. m.p .: 85-95 ° C optical rotation: [α] 20 = -37.83 ° (CHCl 3) D
Synthesis pathway
Trade names
| Country | Trade name | Manufacturer |
|---|---|---|
| Japan | Baynas | Bayer |
| Ukraine | no | no |
Formulations
-
50 mg tablet 75 mg
Reference
-
DE 3631824 (Bayer AG; appl. 19.9.1986; prior. 21.2.1986).
-
EP 728 743 (Bayer AG; appl. 14.2.1996; D-prior. 27.2.1995).
| Patent | Submitted | Granted |
|---|---|---|
| Phenylsulfonamid substituted pyridinealken- and aminooxyalkan-carboxylic-acid derivatives. [EP0471259] | 1992-02-19 | 1995-05-17 |
| Heterocyclic substituted cycloalkano(b)-indolesulfonamides. [EP0473024] | 1992-03-04 | |
| Cycloalkano[b]dihydroindoles and -indolesulphonamides substituted by heterocycles. [EP0451634] | 1991-10-16 | 1994-03-09 |
| Respiratory Drug Condensation Aerosols and Methods of Making and Using Them [US2009258075] | 2009-10-15 | |
| ANTITHROMBOTIC SUBSTITUTED CYCLOALKANO(B)DIHYDROINDOLE- AND -INDOLE-SULPHONAMIDES [US5096897] | 1992-03-17 | |
| Indolesulphonamide-substituted dihydropyridines [US5272161] | 1993-12-21 | |
| THERMODYNAMICALLY STABLE FORM OF (R)-3-[ [(4-FLUOROPHENYL) SULPHONYL]AMINO] -1,2,3,4- TETRAHYDRO -9H-CARBAZOLE -9-PROPANOIC ACID (RAMATROBAN) [WO9933803] | 1999-07-08 |
| DE1695703B2 * | Mar 15, 1967 | Nov 20, 1975 | Sumitomo Chemical Co., Ltd., Osaka (Japan) | Title not available |
| DE2125926A1 * | May 25, 1971 | Jan 27, 1972 | Title not available | |
| DE2226702A1 * | May 25, 1972 | Dec 13, 1973 | Schering Ag | Neue mittel zur behandlung des diabetes mellitus |
| FR1415322A * | Title not available | |||
| GB1487989A * | Title not available | |||
| US4235901 * | May 14, 1979 | Nov 25, 1980 | American Home Products Corporation | 1-Hydroxyalkanamine pyrano(3,4-b)indole compositions and use thereof |
References
- Sugimoto H, Shichijo M, Iino T et al. (April 2003). “An orally bioavailable small molecule antagonist of CRTH2, ramatroban (BAY u3405), inhibits prostaglandin D2-induced eosinophil migration in vitro”. J. Pharmacol. Exp. Ther. 305 (1): 347–52.doi:10.1124/jpet.102.046748. PMID 12649388.
- Royer JF, Schratl P, Carrillo JJ et al. (September 2008). “A novel antagonist of prostaglandin D2 blocks the locomotion of eosinophils and basophils”. Eur. J. Clin. Invest. 38 (9): 663–71.doi:10.1111/j.1365-2362.2008.01989.x. PMID 18837743.
- Fiedler VB, Seuter F, Perzborn E (December 1990). “Effects of the novel thromboxane antagonist Bay U 3405 on experimental coronary artery disease”. Stroke 21 (12 Suppl): IV149–51.PMID 2260140.
- Endo S, Akiyama K (November 1996). “[Thromboxane A2 receptor antagonist in asthma therapy]”. Nippon Rinsho (in Japanese) 54 (11): 3045–8. PMID 8950952.
External links
- (Japanese) Baynas
 |
|
| Systematic (IUPAC) name | |
|---|---|
| 3-((3R)-3-{[(4-fluorophenyl)sulfonyl]amino}-1,2,3,4-tetrahydro-9H-carbazol-9-yl)propanoic acid | |
| Clinical data | |
| Legal status |
|
| Routes | Oral |
| Identifiers | |
| CAS number | 116649-85-5 |
| ATC code | None |
| PubChem | CID 123879 |
| IUPHAR ligand | 1910 |
| ChemSpider | 110413 |
| UNII | P1ALI72U6C  |
| ChEMBL | CHEMBL361812 |
| Chemical data | |
| Formula | C21H21FN2O4S |
| Mol. mass | 416.46 g/mol |
Filed under: Uncategorized Tagged: Bayer AG, Ramatroban