
USV Limited
16-Oct-2014 pub date
WO-2014167577-A2
 PATENT FEATURE ON THIS BLOG
PATENT FEATURE ON THIS BLOG
Title of the invention: “”SYNTHESIS OF DABIGATRAN”.”
Applicants: USV LIMITED (IN).
Inventors: Laxmikant Narhari Patkar (IN), Harish Kashinath Mondkar (IN), Sachin Shivaji Patil (IN), Tanaji Shamrao Jadhav (IN), Nitin Nivrutti Hagavane (IN), Rajesh Ganpat Bopalkar (IN) and Nitin Dnyaneshwar Arote (MY).

The present invention relates to a process for preparation of Dabigatran etexilate or pharmaceutically acceptable salt thereof. The present invention relates to novel compounds, in particular Ethyl-3-{[(2-formyl-l-methyl-lH-benzimidazole-5-yl) carbonyl] -(2-pyridinyl) amino} propanoate and Ethyl-3-{[(2-dichloromethyl-l-methyl -lH-benzimidazole-5-yl)carbonyl]- (2-pyridinyl) amino}propanoate and process for preparation thereof. The present invention further relates to the use of these novel compounds in the preparation of Dabigatran etexilate or pharmaceutically acceptable salt thereof.
Dabigatran is used to prevent strokes in those with atrial fibrillation due to non heart valve causes




Process for preparing dabigatran etexilate mesylate, useful for treating thrombosis, stroke and embolism. Also claims novel intermediates of dabigatran and their synthesis. Represents the first patenting from USV on this API, which was originally developed and launched, by Boehringer Ingelheim for treating conditions such as stroke, thrombosis and atrial fibrillation.
Dabigatran (Pradaxa in Australia, Europe and USA, Pradax in Canada, Prazaxa in Japan) is an oral anticoagulant from the class of the direct thrombin inhibitors. It is being studied for various clinical indications and in some cases it offers an alternative to warfarin as the preferred orally administered anticoagulant (“blood thinner”) since it does not require frequent blood tests for international normalized ratio (INR) monitoring while offering similar results in terms of efficacy. There is no specific way to reverse the anticoagulant effect of dabigatran in the event of a major bleeding event, unlike warfarin, although a potential dabigatran antidote (pINN: idarucizumab) is undergoing clinical studies. It was developed by the pharmaceutical company Boehringer Ingelheim.
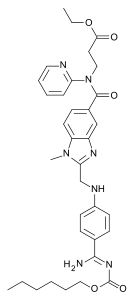
Family members of its product case, WO9837075, have SPC protection in most EU states until February 2023, and expiry dates in the US until July 2020. The FDA Orange Book lists US7932273 (product derivative) and US7866474 (describing film blister-card containers for pradaxa®), which expire in September 2025 and August 2027 respectively, for dabigatran.
The drug also has New Chemical Entity exclusivity expiring on October 19, 2015. As of October 2014, Newport Premium™ reports that USV has dabigatran under development.
SEE
РЕФЕРАТ WO2014167577
요약서 WO2014167577
要約書 WO2014167577
摘要 WO2014167577
Filed under: PATENT, PATENTS Tagged: dabigatran, NEW PATENT, usv, WO 2014167577