Gemeprost, SC-37681, Ono-802, Cergem, Preglandin, Cervagem,
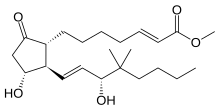
(E) -7 – [(1R, 2R, 3R-3-Hydroxy-2 – [(E) - (3R) -3-hydroxy-4,4-dimethyl-1-octenyl] -5-oxocyclopentyl] -2 -heptenoic acid methyl ester;
16,16-Dimethyl-DELTA2-trans-PGE1 methyl ester;
9-Oxo-11alpha, 15alpha-dihydroxy-16,16-dimethyl-2-trans, 13-trans-prostadiene-1-oic acid
Gemeprost (16, 16-dimethyl-trans-delta2 PGE1 methyl ester) is an analogue of prostaglandin E1.
Clinical use
It is used as a treatment for obstetric bleeding.
It is used with mifepristone to terminate pregnancy up to 24 weeks gestation. [1]
Side effects
Vaginal bleeding, cramps, nausea, vomiting, loose stools or diarrhea, headache, muscle weakness; dizziness; flushing; chills; backache; dyspnoea; chest pain; palpitations and mild pyrexia. Rare: Uterine rupture, severe hypotension, coronary spasms with subsequent myocardial infarctions
 |
|
| Systematic (IUPAC) name | |
|---|---|
| methyl (2E,11α,13E,15R)-11,15-dihydroxy-16,16-dimethyl-9-oxoprosta-2,13-dien-1-oate | |
| Clinical data | |
| AHFS/Drugs.com | International Drug Names |
| Legal status | ? |
| Routes | Pessary |
| Identifiers | |
| CAS number | 64318-79-2 |
| ATC code | G02AD03 |
| PubChem | CID 5282237 |
| ChemSpider | 4445416  |
| UNII | 45KZB1FOLS  |
| KEGG | D02073  |
| Synonyms | methyl (E)-7-[(1R,2S,3R)-3-hydroxy-2-[(E,3R)-3-hydroxy-4,4-dimethyl-oct-1-enyl]-5-oxo-cyclopentyl]hept-2-enoate |
| Chemical data | |
| Formula | C23H38O5 |
| Mol. mass | 394.545 g/mol |
………………………………
http://www.chemdrug.com/databases/8_0_oqxuqtwlqgeukaaa.html

The reaction of 3-bromopropionic acid (I) with triphenylphosphine (II) in refluxing acetonitrile gives (2-carboxyethyl) -triphenylphosphonium bromide (III), which by a Wittig reaction with 2-oxa-3-hydroxy-6-syn- ( 3alpha-tetrahydropyranyloxy-4,4-dimethyl-1-trans-octen-1-yl) -7-anti-tetrahydropyranyloxybicyclo- [3.3.0] cis-octane (IV) (prepared according to reference 2) by means of sodium dimethylsulfinate in DMSO yields 9alpha-hydroxy-11alpha, 15alpha-bis (tetrahydropyranyloxy) -16,16-dimethyl-alpha-dinorprosta-5-cis-13-trans-dienoic acid (V). The reduction of (V) with H2 over Pd / C in methanol affords the 13-trans-prostenoic acid (VI), which is methylated with CH2N2 in ether yielding the methyl ester (VII). The reduction of (VII) with diisobutyl aluminum hydride in toluene affords the corresponding aldehyde (VIII) , which by a Wittig reaction with triethyl phosphonoacetate (IX) by means of NaH in THF is converted into 9alpha-hydroxy-11alpha, 15alpha-bis (tetrahydropyranyloxy) -16,16-dimethylprosta-2-trans-dienoic acid ethyl ester (X .) The hydrolysis of the ester (X) with KOH in ethanol-water gives the corresponding acid (XI), which is oxidized with CrO3, MnSO4 and H2SO4 in ether – water yielding the protected ketoacid (XII) The hydrolysis of (XII. ) with acetic acid-water at 80 C gives 9-oxo-11alpha, 15alpha-dihydroxy-16,16-dimethyl-prosta-2-trans-13-trans-dienoic acid (16,16-dimethyl-DELTA2-trans-PGE1 ) (XIII), which is finally methylated with CH2N2 in ether
References
- Bartley J, Brown A, Elton R, Baird DT (October 2001). “Double-blind randomized trial of mifepristone in combination with vaginal gemeprost or misoprostol for induction of abortion up to 63 days gestation”. Human reproduction (Oxford, England) 16 (10): 2098–102.doi:10.1093/humrep/16.10.2098. PMID 11574498. Retrieved 2008-10-29.
 |
|
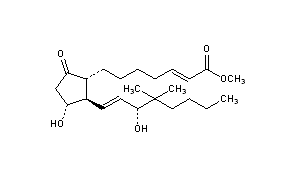
: Gemeprost
CAS 64318-79-2
CAS Name: (2E,11a,13E,15R)-11,15-Dihydroxy-16,16-dimethyl-9-oxoprosta-2,13-dien-1-oic acid methyl ester
Additional Names: 16,16-dimethyl-trans-D2-PGE1 methyl ester
Manufacturers’ Codes: ONO-802
Trademarks: Cergem (Searle); Cervagem(e) (M & B); Preglandin (Ono)
Molecular Formula: C23H38O5
Molecular Weight: 394.54
Percent Composition: C 70.02%, H 9.71%, O 20.28%
Literature References:
Analog of prostaglandin E1, q.v. Prepn: M. Hayashi et al., DE 2700021; eidem, US 4052512 (both 1977 to Ono);
H. Suga et al., Prostaglandins 15, 907 (1978).
Effects on uterine contractility and steroid hormone plasma levels: K. Oshimaet al., J. Reprod. Fertil. 55, 353 (1979).
Effects on reproductive function: K. Matsumoto et al., Nippon Yakurigaku Zasshi 79, 15 (1982), C.A. 96, 98392 (1982).
Use in termination of first trimester pregnancy: O. Reiertsen et al., Prostaglandins Leukotrienes Med. 8, 31 (1982).
Therap-Cat: Abortifacient; oxytocic.
Keywords: Abortifacient/Interceptive; Oxytocic; Prostaglandin/Prostaglandin Analog
|
Filed under: Uncategorized Tagged: gemeprost, gemoprost
