losmapimod
Losmapimod is a p38 mitogen-activated protein kinase inhibitor.
Smithkline Beecham Corporation
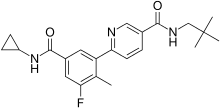
6-[5-(cyclopropylcarbamoyl)-3-fluoro-2-methylphenyl]-N-(2,2-dimethylpropyl)pyridine-3-carboxamide
cas 585543-15-3
Synonym: Losmapimod; GW856553; GW-856553; GW 856553)
IUPAC/Chemical name:
6-[5-(cyclopropylcarbamoyl)-3-fluoro-2-methylphenyl]-N-(2,2-dimethylpropyl)pyridine-3-carboxamide
GlaxoSmithKline has begun a Phase III study cardiovascular outcomes study of its investigational compound losmapimod in patients with acute coronary syndrome.
The trial will assess whether losmapimod can reduce the risk of a subsequent cardiac event when administered orally twice a day for three months immediately after presentation with an ACS, such as heart attack. GSK says that some 25,500 patients will be enrolled over the study period across 39 countries.
Read more at: http://www.pharmatimes.com/Article/14-06-06/GSK_launches_huge_Phase_III_trial_for_heart_drug_losmapimod.aspx#ixzz33vFHAK14
Losmapimod, also know as GW856553 or GW856553X, is a drug developed by GlaxoSmithKline which acts as a selective inhibitor of the enzyme family known as p38 mitogen-activated protein kinases. p38 mitogen-activated protein kinases are mediators of inflammation. A Phase II human clinical trial for the treatment of COPD (chronic obstructive pulmonary disease) is underway. Inhibiting these enzymes has been shown to produce antidepressant and antipsychotic effects in animal studies, with the mechanism thought to involve increased neurogenesis probably related to BDNF release. Losmapimod has completed Phase II human clinical trials for the treatment of depression although its safety and efficacy have yet to be proven in further trials. Losmapimod is also being studied for cardiovascular disease. A Phase II trial to study its effects in myocardial infarction (heart attack) is ongoing.
Losmapimod (GW856553X) is a drug developed by GlaxoSmithKline which acts as a selective inhibitor of the enzyme family known as p38 mitogen-activated protein kinases.[1]
p38 mitogen-activated protein kinases are mediators of inflammation. A Phase II human clinical trial for the treatment of COPD(chronic obstructive pulmonary disease)[2] is underway. Inhibiting these enzymes has been shown to produce antidepressant andantipsychotic effects in animal studies, with the mechanism thought to involve increased neurogenesis[3] probably related to BDNFrelease. Losmapimod has completed Phase II human clinical trials for the treatment of depression although its safety and efficacy have yet to be proven in further trials.[4]
Losmapimod is also being studied for cardiovascular disease.[5] A Phase II trial to study its effects in myocardial infarction (heart attack) is ongoing.[6]
………………………
http://www.google.com/patents/US8252818

| Example 36 6-(5- Cyclopropylcarbamoyl- 3-fluoro-2-methyl- phenyl)-N-(2,2- dimethylpropyl)- nicotinamide | 6-Chloro-N-(2,2- dimethylpropyl))nicotin- amide (Intermediate 24) | 384 | 3.01 |
……………….
https://www.google.com/patents/US7514456
General Method A
6-Bromonicotinic acid (100 mg, 0.5 mmol) was heated at 95° C. in thionyl chloride (0.63 ml) for 2 hours. The excess thionyl chloride was evaporated under vacuum and the residue dissolved in DCM (2 ml). To this solution, amine (0.5 mmol) and sodium carbonate (100 mg) were added and the reaction was stirred at room temperature for 2 hours. The reaction was filtered and the residue washed with DCM. The combined filtrate and washings were reduced to dryness to give the desired 6-chloronicotinamide.
| Retention time | |||
| Compound | Amine | MH+ | (minutes) |
| Intermediate 22: 6-Chloro-N-(3- | 3-methylbutylamine | 227 | 2.92 |
| methylbutyl)nicotinamide | |||
| Intermediate 23: 6-Chloro-N-(1- | 1-cyclopropylethylamine | 225 | 2.65 |
| cyclopropylethyl)nicotinamide | |||
| Intermediate 24: 6-Chloro-N-(2,2- | 2,2-dimethylpropylamine | 227 | 2.82 |
| dimethylpropyl))nicotinamide | |||
| Intermediate 25: 6-Chloro-N-(2,2- | 2,2- | 225 | 2.67 |
|
8-29-2012
|
Nicotinamide derivatives useful as P38 inhibitors
|
|
|
8-3-2011
|
Use of a p38 Kinase Inhibitor for Treating Psychiatric Disorders
|
|
|
11-24-2010
|
3-Aminocarbonyl, 6-phenyl substituted pyridine-1-oxides as p38 kinase inhibitors
|
|
|
8-27-2010
|
NICOTINAMIDE DERIVATES USEFUL AS P38 INHIBITORS
|
|
|
5-5-2010
|
Nicotinamide Derivatives Useful as p38 Inhibitors
|
|
|
4-8-2009
|
Nicotinamide Derivatives Useful as p38 Inhibitors
|
|
|
10-25-2006
|
Nicotinamide derivatives useful as p38 inhibitors.
|
References
- Aston N, Bamborough P, Buckton J, Edwards C, Holmes D, Jones K, Patel V, Smee P, Somers D, Vitulli G, Walker A. p38α Mitogen-Activated Protein Kinase Inhibitors: Optimization of a Series of Biphenylamides to Give a Molecule Suitable for Clinical Progression.Journal of Medicinal Chemistry 2009, 52(20), 6257. doi:10.1021/jm9004779
- Randomised, Double-Blind, Placebo-Controlled, Parallel-Group, Multi-centre, Dose Ranging Study to Evaluate the Efficacy and Safety of Losmapimod Tablets Administered Twice Daily Compared With Placebo for 24 Weeks in Adult Subjects With Chronic Obstructive Pulmonary Disease (COPD)
- Noh JS, Kang HJ, Kim YE, Sohn S, Chung YK, Kim SU, Gwag BJ. Haloperidol-Induced Neuronal Apoptosis: role of p38 and c-Jun-NH(2)-terminal protein kinase. Journal of Neurochemistry 2000, 75(6), 2327. PMID 11080184 doi:10.1046/j.1471-4159.2000.0752327.x
- A Study of GW856553X For the Treatment of Depression
- Cheriyan et al., Circulation 2011, 123(5), 515-523. Inhibition of p38 Mitogen-Activated Protein Kinase Improves Nitric Oxide–Mediated Vasodilatation and Reduces Inflammation in Hypercholesterolemia doi:10.1161/CIRCULATIONAHA.110.971986
- A Study to Evaluate the Safety of 12 Weeks of Dosing With GW856553 and Its Effects on Inflammatory Markers, Infarct Size, and Cardiac Function in Subjects With Myocardial Infarction Without ST-segment Elevation (Solstice)
|
more References |
1: Yang S, Beerahee M. Losmapimod concentration-QT relationship in healthy volunteers: meta-analysis of data from six clinical trials. Eur J Clin Pharmacol. 2013 Jun;69(6):1261-7. doi: 10.1007/s00228-012-1469-1. Epub 2013 Jan 17. PubMed PMID: 23325437.
2: Yang S, Lukey P, Beerahee M, Hoke F. Population pharmacokinetics of losmapimod in healthy subjects and patients with rheumatoid arthritis and chronic obstructive pulmonary diseases. Clin Pharmacokinet. 2013 Mar;52(3):187-98. doi: 10.1007/s40262-012-0025-6. PubMed PMID: 23254770.
3: Dewenter M, Vettel C, El-Armouche A. [Losmapimod: a novel drug against cardiovascular diseases?]. Dtsch Med Wochenschr. 2013 Jan;138(1-2):39-42. doi: 10.1055/s-0032-1327368. Epub 2012 Dec 18. Review. German. PubMed PMID: 23250695.
4: Ostenfeld T, Krishen A, Lai RY, Bullman J, Baines AJ, Green J, Anand P, Kelly M. Analgesic efficacy and safety of the novel p38 MAP kinase inhibitor, losmapimod, in patients with neuropathic pain following peripheral nerve injury: a double-blind, placebo-controlled study. Eur J Pain. 2013 Jul;17(6):844-57. doi: 10.1002/j.1532-2149.2012.00256.x. Epub 2012 Dec 14. PubMed PMID: 23239139.
5: Barbour AM, Sarov-Blat L, Cai G, Fossler MJ, Sprecher DL, Graggaber J, McGeoch AT, Maison J, Cheriyan J. Safety, tolerability, pharmacokinetics and pharmacodynamics of losmapimod following a single intravenous or oral dose in healthy volunteers. Br J Clin Pharmacol. 2013 Jul;76(1):99-106. doi: 10.1111/bcp.12063. PubMed PMID: 23215699; PubMed Central PMCID: PMC3703232.
6: Melloni C, Sprecher DL, Sarov-Blat L, Patel MR, Heitner JF, Hamm CW, Aylward P, Tanguay JF, DeWinter RJ, Marber MS, Lerman A, Hasselblad V, Granger CB, Newby LK. The study of LoSmapimod treatment on inflammation and InfarCtSizE (SOLSTICE): design and rationale. Am Heart J. 2012 Nov;164(5):646-653.e3. doi: 10.1016/j.ahj.2012.07.030. Epub 2012 Oct 16. PubMed PMID: 23137494.
7: Elkhawad M, Rudd JH, Sarov-Blat L, Cai G, Wells R, Davies LC, Collier DJ, Marber MS, Choudhury RP, Fayad ZA, Tawakol A, Gleeson FV, Lepore JJ, Davis B, Willette RN, Wilkinson IB, Sprecher DL, Cheriyan J. Effects of p38 mitogen-activated protein kinase inhibition on vascular and systemic inflammation in patients with atherosclerosis. JACC Cardiovasc Imaging. 2012 Sep;5(9):911-22. doi: 10.1016/j.jcmg.2012.02.016. PubMed PMID: 22974804.
8: Lomas DA, Lipson DA, Miller BE, Willits L, Keene O, Barnacle H, Barnes NC, Tal-Singer R; Losmapimod Study Investigators. An oral inhibitor of p38 MAP kinase reduces plasma fibrinogen in patients with chronic obstructive pulmonary disease. J Clin Pharmacol. 2012 Mar;52(3):416-24. doi: 10.1177/0091270010397050. Epub 2011 Nov 16. PubMed PMID: 22090363.
9: Cheriyan J, Webb AJ, Sarov-Blat L, Elkhawad M, Wallace SM, Mäki-Petäjä KM, Collier DJ, Morgan J, Fang Z, Willette RN, Lepore JJ, Cockcroft JR, Sprecher DL, Wilkinson IB. Inhibition of p38 mitogen-activated protein kinase improves nitric oxide-mediated vasodilatation and reduces inflammation in hypercholesterolemia. Circulation. 2011 Feb 8;123(5):515-23. doi: 10.1161/CIRCULATIONAHA.110.971986. Epub 2011 Jan 24. PubMed PMID: 21262998.
10: Welchman R. Advances and Progress in Drug Design – SMi’s ninth annual meeting. IDrugs. 2010 Apr;13(4):239-42. PubMed PMID: 20373252.
11: Willette RN, Eybye ME, Olzinski AR, Behm DJ, Aiyar N, Maniscalco K, Bentley RG, Coatney RW, Zhao S, Westfall TD, Doe CP. Differential effects of p38 mitogen-activated protein kinase and cyclooxygenase 2 inhibitors in a model of cardiovascular disease. J Pharmacol Exp Ther. 2009 Sep;330(3):964-70. doi: 10.1124/jpet.109.154443. Epub 2009 Jun 25. PubMed PMID: 19556450.
GSK Announces Phase III Cardiovascular Outcomes Study with Losmapimod in Patients with Acute Coronary Syndrome
GlaxoSmithKline plc Thursday 5 June 2014, London UK (LSE/NYSE: GSK) today announced the start of a pivotal phase III study, LATITUDE-TIMI 60, to evaluate the effects of losmapimod in patients presenting with acute coronary syndrome. The global, phase III study will assess whether losmapimod can reduce the risk of a subsequent cardiac event when administered…
Filed under: Phase3 drugs, Uncategorized Tagged: losmapimod


