
Ezatiostat
168682-53-9 (Ezatiostat); 286942-97-0 (Ezatiostat HCl salt)
gamma-Glu-S-BzCys-PhGly diethyl ester
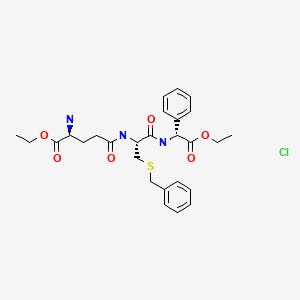
Ezatiostat hydrochloride
Target: glutathione S-transferase P1-1 (GSTP1-1) inhibitor
Pathway: hsa00480 Glutathione metabolism
Activity: Treatment of disorders of bone marrow cellular growth and differentiation
see http://www.ncbi.nlm.nih.gov/pubmed?term=TLK-199&cmd=search
| Telik, Inc. |
innovator
TLK199; TLK-199; TLK 199; Brand name: TELINTRA®。ethyl (2R)-[(4S)-4-amino-5-ethoxy-5-oxopentanoyl]-S-benzyl-L-cysteinyl-2- phenylglycinate.
ethyl (2S)-2-amino-5-[[(2R)-3-benzylsulfanyl-1-[[(1R)-2-ethoxy-2-oxo-1-phenylethyl]amino]-1-oxopropan-2-yl]amino]-5-oxopentanoate.
IUPAC/Chemical name:
(S)-ethyl 2-amino-5-(((R)-3-(benzylthio)-1-(((S)-2-ethoxy-2-oxo-1-phenylethyl)amino)-1-oxopropan-2-yl)amino)-5-oxopentanoate
C27H35N3O6S
Exact Mass: 529.2246
nmr.http://www.medkoo.com/Product-Data/Ezatiostat/ezatiostat-QC-CRB40225web.pdf
Telintra is a small molecule product candidate designed to stimulate the production of blood cells in the bone marrow. Many conditions are characterized by depleted bone marrow, including myelodysplastic syndrome, a form of pre-leukemia in which the bone marrow produces insufficient levels of one or more of the 3 major blood elements (white blood cells, red blood cells and platelets). A reduction in blood cell levels is also a common, toxic effect of many standard chemotherapeutic drugs.
Ezatiostat is a liposomal small-molecule glutathione analog inhibitor of glutathione S-transferase (GST) P1-1 with hematopoiesis-stimulating activity. After intracellular de-esterification, the active form of ezatiostat binds to and inhibits GST P1-1, thereby restoring Jun kinase and MAPK pathway activities and promoting MAPK-mediated cellular proliferation and differentiation pathways. This agent promotes the proliferation and maturation of hematopoietic precursor cells, granulocytes, monocytes, erythrocytes and platelets
Phase II trial myelodysplastic syndrome (MDS): Cancer. 2012 Apr 15;118(8):2138-47.
Phase I trial myelodysplastic syndrome (MDS): J Hematol Oncol. 2012 Apr 30;5:18. doi: 10.1186/1756-8722-5-18; Blood. 2009 Jun 25;113(26):6533-40; J Hematol Oncol. 2009 May 13;2:20.
Ezatiostat hydrochloride is the hydrochloride acid addition salt of ezatiostat. Ezatiostat, also known as TLK199 or TER 199, is a compound of the formula:
Ezatiostat has been shown to induce the differentiation of HL-60 promyelocyte leukemia cells in vitro, to potentiate the activity of cytotoxic agents both in vitro and in vivo, and to stimulate colony formation of all three lineages of hematopoietic progenitor cells in normal human peripheral blood.
In preclinical testing, ezatiostat has been shown to increase white blood cell production in normal animals, as well as in animals in which white blood cells were depleted by treatment with cisplatin or fluorouracil. Similar effects may provide a new approach to treating myelodysplastic syndrome (MDS).
Many conditions, including MDS, a form of pre-leukemia in which the bone marrow produces insufficient levels of one or more of the three major blood elements (white blood cells, red blood cells, and platelets), are characterized by depleted bone marrow. Myelosuppression, which is characterized by a reduction in blood cell levels and in a reduction of new blood cell generation in the bone marrow, is also a common, toxic effect of many standard chemotherapeutic drugs.
Ezatiostat hydrochloride in a liposomal injectable formulation was studied in a clinical trial for the treatment of MDS, and results from this trial, reported by Raza et al., J Hem. One, 2:20 (published online 13 May 2009), demonstrated that administration of TLK199 was well tolerated and resulted in multi-lineage hematologic improvement.
Ezatiostat hydrochloride in a tablet formulation has been evaluated in a clinical trial for the treatment of MDS, as reported by Raza et al., Blood, 113:6533-6540 (prepublished online 27 April 2009) and a single-patient report by Quddus et al., J Hem. One, 3:16 (published online 23 April 2010), and is currently being evaluated in clinical trials for the treatment of MDS and for severe chronic idiopathic neutropenia.
When used for treating humans, it is important that a crystalline therapeutic agent like ezatiostat hydrochloride retains its polymorphic and chemical stability, solubility, and other physicochemical properties over time and among various manufactured batches of the agent. If the physicochemical properties vary with time and among batches, the administration of a therapeutically effective dose becomes problematic and may lead to toxic side effects or to ineffective therapy, particularly if a given polymorph decomposes prior to use, to a less active, inactive, or toxic compound.
Therefore, it is important to choose a form of the crystalline agent that is stable, is manufactured reproducibly, and has physicochemical properties favorable for its use as a therapeutic agent.
Ezatiostat hydrochloride (USAN) has the molecular weight of 566.1, the trademark of Telintra®, and the CAS registry number of 286942-97-0. Ezatiostat hydrochloride has been evaluated for the treatment of myelodysplastic syndrome (MDS), in a Phase I-IIa study using a liposomal formulation (U.S. Pat. No. 7,029,695), as reported at the 2005 Annual Meeting of the American Society for Hematology (Abstract #2250) and by Raza et al. in Journal of Hematology & Oncology, 2:20 (published online on 13 May 2009); and in a Phase I study using a tablet formulation, as reported at the 2007 Annual Meeting of the American Society for Hematology (Abstract #1454) and by Raza et al. in Blood, 113:6533-6540 (prepublished online on 27 Apr. 2009), and in a single patient case report by Quddus et al. in Journal of Hematology & Oncology, 3:16 (published online on 23 Apr. 2010).
…………………………………………………………………
http://www.google.com/patents/US20110301376
Preparation of Ezatiostat Hydrochloride
In another aspect, this invention provides a process comprising the steps of contacting a compound of formula:
or a salt thereof with a compound of formula:
or a salt thereof and an activating agent under conditions which provide a compound of formula:
In one embodiment, the process further comprises deprotecting the compound of formula:
under conditions which provide a compound of formula:
or a salt thereof. In another embodiment, the compound provided is ezatiostat hydrochloride.
In another aspect, this invention provides a process comprising contacting a compound of formula:
or a salt thereof with an ethylating agent under conditions which provide a compound of formula:
In another embodiment, the process further comprises debenzylating the compound of formula:
under conditions which provide a compound of formula:
or a salt thereof.
In another aspect, this invention provides a process comprising the steps of contacting a compound of formula:
or a salt thereof having a t-butoxycarbonyl group with an activating agent and a compound of formula:
or a salt thereof under conditions which provide a compound of formula:
In another embodiment, the process further comprises deprotecting the tertiarybutyloxycarboyl (Boc) group under conditions to provide a compound of formula:
or a salt thereof.
Certain preferred embodiments of this invention are illustrated in the reaction scheme and described below. In the peptide coupling the amino acid reagents are used generally at a 1:1 molar ratio, and the activating reagent (isobutyl chloroformate) and the base (NMM) are used in slight excess over the amino acid reagents; while in the esterification of the N-BOC-L-glutamic acid γ-benzyl ester the esterifying agent (diethyl sulfate) and base are used in about 1.4-fold excess.
EXAMPLESAs relevant and unless otherwise noted, all operations were conducted under nitrogen purge and with stirring. Water was osmosis purified, and solvents were filtered. Unless otherwise stated, all temperatures are in degrees Celcius (° C.) and the following abbreviations have the following definitions:Et EthylHCl(g) HCl gasN-BOC or N-Boc N-tertiarybutyloxycarbonylL LiterKg KilogramNMM N-methylmorpholineMol Molew/w weight by weightExample 1Preparation of S-benzyl-L-cysteinyl-D-phenylglycine ethyl ester hydrochloride (3)Without stirring, 45.1 Kg N-BOC-S-benzyl-L-cysteine (1) was added to a 600 L jacketed glass-lined reactor, followed by 45 L ethyl acetate. Stirring was started and the temperature was reduced to 13° C. NMM, 15.3 Kg, was added over 50 minutes, and rinsed in with 6 L ethyl acetate, and stirring stopped. Ethyl acetate, 315 L, was added to an 800 L cooled jacketed glass-lined reactor, followed by 20.7 Kg isobutyl chloroformate, rinsed in with 11 L ethyl acetate, and the mixture cooled to −10° C. The N-BOC-S-benzyl-L-cysteine NMM salt solution was added to the 800 L reactor over 5 hours, its reactor rinsed with 11 L ethyl acetate, and the rinse solution added to the 800 L reactor, while maintaining the temperature at (−10˜−7)° C. D-Phenylglycine ethyl ester hydrochloride, 31.2 Kg, was added in 8 portions over 50 minutes, followed by 15.3 Kg NMM in 8 portions over 1.3 hours, rinsed in with 2×5 L portions of ethyl acetate, allowing the mixture to warm to −1° C. by the end of the addition. The mixture was gradually warmed to 1° C. for 30 minutes, then to 20° C. over 2 hours, and maintained at (20˜25)° C. for 5 hours. The reaction mixture was washed twice with water: the first time adding 66 L water, stirring at room temperature for 40 minutes, allowing the phases to separate for 30 minutes, then removing the aqueous phase; the second time adding 68 L water, bringing the pH to 1.9 with the addition of 0.45 L 36% hydrochloric acid, stirring at room temperature for 35 minutes, allowing the phases to separate for 1 hour, then removing the aqueous phase. The organic phase was then heated to 38° C., and the pressure reduced to about 0.25 bar until no further gas was released, then to about (0.07-0.1) bar and solvents removed by distillation until 266 L of distillate had been removed. Four cycles of addition of 45 L ethyl acetate and removal of 45 L solvent by distillation were performed, and the water content of the remaining mixture was checked to ensure that it was below 0.1%. With the mixture at 36° C., 194 L heptanes was added, maintaining the temperature about 36° C., and held at that temperature for 2.3 hours. A further 194 L heptanes was added, allowing the temperature to cool to 30° C., and the temperature then reduced to −1° C. over 2.3 hours and then to −5° C. over 1 hour, and N-BOC-S-benzyl-L-cysteinyl-D-phenylglycine ethyl ester recovered by filtration, washing twice with 30 L each of heptanes at −5° C., giving 85 Kg (63 Kg dry basis) N-BOC-S-benzyl-L-cysteinyl-D-phenylglycine ethyl ester. Without stirring, the damp N-BOC-S-benzyl-L-cysteinyl-D-phenylglycine ethyl ester was loaded into an 800 L jacketed glass-lined reactor, followed by 257 L ethyl acetate. Stirring was started and the temperature brought to 22° C., then the nitrogen purge stopped and 12.2 Kg hydrogen chloride gas was added through an immersion tube over 1.8 hours, allowing the temperature to increase to 38° C. The temperature was increased to 41° C., and the mixture held at that temperature for 9 hours. About 280 L of solvents were removed by distillation at that temperature and a pressure of (0.2˜0.1) bar over about 2 hours. Two cycles of addition of ethyl acetate and removal of solvent by distillation were performed, using 52 L in the first cycle and 77 L in the second cycle, and the viscous solution of S-benzyl-L-cysteinyl-D-phenylglycine ethyl ester hydrochloride (3) in ethyl acetate, 148 Kg, was cooled to room temperature and filtered into a storage drum.Example 2Preparation of N-BOC-L-glutamic acid α-ethyl ester (6)Without stirring, 41 Kg N-BOC-L-glutamic acid γ-benzyl ester (4) was added to an 800 L jacketed glass-lined reactor, followed by 2.5 L water and 123 L ethyl acetate. The mixture was then stirred until the N-BOC-L-glutamic acid γ-benzyl ester completely dissolved, keeping the temperature below 15° C. Potassium carbonate fine powder, 23.4 Kg, was added in five batches, and the mixture then heated to 55° C. and maintained at that temperature for 40 minutes, giving a heterogeneous and completely fluid mixture. Diethyl sulfate, 26.2 Kg, was added over 2 hours, and rinsed in with 5 L ethyl acetate, with the temperature remaining at about 52° C. The nitrogen purge was stopped and a solution of 20 Kg ammonium chloride in 73 L water at room temperature added over 2 hours to the mixture, maintaining the temperature near 50° C., then rinsing in with 10 L water. Nitrogen purging was resumed, and the mixture was maintained at about 50° C. for 3 hours, then lowered to about 45° C., the stirring stopped, the phases allowed to separate for 30 minutes, and the lower, aqueous, phase removed. The organic phase, containing N-BOC-L-glutamic acid γ-benzyl α-ethyl ester (5), was washed three times with water, each time adding 41 L water, stirring at room temperature for 30 minutes, allowing the phases to separate for 30 minutes, then removing the aqueous phase. The organic phase was heated to 35° C., and the pressure reduced, starting at about 0.2 bar and reducing as necessary until 82 Kg solvent had been removed by distillation, leaving about (70˜80) L of slightly opalescent solution. This solution was heated to 53° C., and 102 L heptanes was added, maintaining the same temperature. The solution was then filtered, rinsing with a further 13 L heptanes, then cooled to 32° C. to cause crystallization and maintained at that temperature for 1 hour. A further 66 L heptanes was added, and the mixture cooled to 22° C. and held for 1 hour, then cooled to −5° C. and held for another 1 hour. The mixture was then filtered to isolate the N-BOC-L-glutamic acid γ-benzyl α-ethyl ester (5), which was washed twice, each time with 25 L heptanes cooled to (−5˜0)° C., and dried under vacuum at 40° C., giving 39.3 Kg N-BOC-L-glutamic acid γ-benzyl α-ethyl ester (5).A 4000 L hydrogenator was purged with nitrogen, then under nitrogen sweep and no stirring loaded with 39.2 Kg N-BOC-L-glutamic acid γ-benzyl α-ethyl ester (5), 2.0 Kg 5% palladium on carbon, and 432 L ethyl acetate, and purged (3 bar) and decompressed (0.2 bar) twice with nitrogen and twice with hydrogen. Stirring was begun and the mixture heated to (37±2)° C., hydrogenated at that temperature under 2.8 bar hydrogen pressure until no further hydrogen absorption occurred, then held under 2.8 bar hydrogen pressure for 12 hours. Completion of hydrogenation was confirmed by thin-layer chromatography of a sample. The mixture was cooled to 28° C., the hydrogen purged from the hydrogenator, and the hydrogenator purged (2 bar) and decompressed (0.2 bar) twice with nitrogen. The mixture was filtered through a filter precoated with 10 Kg powdered cellulose in 200 L ethyl acetate, then the filter washed with the ethyl acetate used to form the precoat, giving a total of 626 Kg of a dilute ethyl acetate solution containing 29.5 Kg N-BOC-L-glutamic acid α-ethyl ester (6). This was distilled at (35˜40)° C. and (0.16˜0.18) bar to give 67 L of concentrated solution, then 29 L of ethyl acetate added and the solution redistilled to again give 67 L of concentrated solution.Example 3Preparation of Ezatiostat HydrochlorideThe concentrated solution of N-BOC-L-glutamic acid α-ethyl ester (6), 61.2 Kg (containing 27.8 Kg N-BOC-L-glutamic acid α-ethyl ester), was added to a 600 L jacketed glass-lined reactor, rinsed in with 5 L ethyl acetate, then cooled to 14° C. NMM, 10.8 Kg, was added over 50 minutes and rinsed in with 5 L ethyl acetate, then stirring stopped, giving an ethyl acetate solution of N-BOC-L-glutamic acid α-ethyl ester NMM salt. Ethyl acetate, 475 L, was added to a 1300 L cooled jacketed glass-lined reactor, followed by 14.5 Kg isobutyl chloroformate, rinsed in with 2×10 L ethyl acetate, and the mixture cooled to −11° C. The N-BOC-L-glutamic acid α-ethyl ester NMM salt solution was added to the 1300 L reactor over 1.3 hours, its reactor rinsed with 10 L ethyl acetate, and the rinse solution added to the 1300 L reactor, then stirred for an additional 30 minutes, while maintaining the temperature at about −13° C.S-benzyl-L-cysteinyl-D-phenylglycine ethyl ester hydrochloride (3) in ethyl acetate, 112 Kg (containing 41.3 Kg S-benzyl-L-cysteinyl-D-phenylglycine ethyl ester hydrochloride) was added in 4 portions over 45 minutes, and rinsed in with 5 L ethyl acetate, followed by 10.8 Kg NMM in 8 portions over 1.3 hours, rinsed in with 2×5 L portions of ethyl acetate, allowing the mixture to warm to −4° C. by the end of the addition. The mixture was gradually warmed to 30° C. over 2 hours, and maintained at (30˜35)° C. for 2 hours. The reaction mixture was washed twice with water: the first time adding 100 L water, heating to 41° C., allowing the phases to separate for 30 minutes, then removing the aqueous phase; the second time adding 100 L water, bringing the pH to 2.0 with the addition of 0.8 L 36% hydrochloric acid, stirring at 43° C. for 30 minutes, allowing the phases to separate for 1 hour, then removing the aqueous phase. The organic phase was then heated to 42° C., and the pressure reduced to about 0.25 bar until no further gas was released and solvents removed by distillation until 495 L of distillate had been removed. Four cycles of addition of 120 L ethyl acetate and removal of 120 L solvent by distillation were performed, and the water content of the remaining mixture was checked to ensure that it was below 0.1%. With the mixture at 42° C., 610 L of ethyl acetate was added, maintaining the temperature about 41° C., then heating to 58° C. to ensure dissolution. The solution was filtered, rinsing the filter with 18 L ethyl acetate, and the solution allowed to cool to 22° C. The nitrogen purge was stopped and 22.2 Kg hydrogen chloride gas was added through an immersion tube over 2 hours, then the mixture held at that temperature for 2 hours. The mixture was heated to 31° C. over 1.5 hours, and held at about that temperature for 15.5 hours. Solvents were removed by distillation at 33° C. and a pressure of about 0.13 bar over about 1.5 hours to give a volume of concentrated solution of about 630 L. Ethyl acetate, 100 L, was added, and the mixture cooled to 25° C. and held at that temperature for 30 minutes. The crude ezatiostat hydrochloride was recovered by filtration and washed with 30 L ethyl acetate, giving 113 Kg damp crude ezatiostat hydrochloride, which was dried at 40° C. under vacuum for 24 hours to give 52.8 Kg dry crude ezatiostat hydrochloride.Example 4Crystallization of Ezatiostat Hydrochloride to Form Pure Crystalline Ezatiostat Hydrochloride Ansolvate Form D61.5 Kg crude ezatiostat hydrochloride was added to a reactor at room temperature, followed by 399 liter (L) ethanol, and this mixture was heated to 68° C. to completely dissolve the ezatiostat hydrochloride, filtered, then allowed to cool to 65° C. and checked for clarity and the absence of crystallization. About 1.3 Kg of ezatiostat hydrochloride ansolvate form D was suspended in 9 L of ethyl acetate, and about one-half of this suspension was added to the ethanol solution. The mixture was cooled to 63° C. and the second half of the suspension added to the mixture. The resulting mixture was cooled gradually to 45° C., 928 L ethyl acetate was added, and the mixture was cooled to 26° C. and held at about that temperature for about 5 hours, then cooled to −2° C. The mixture, containing crystalline ezatiostat hydrochloride ansolvate, was filtered, and the residue washed twice with 65 L of chilled (0-5° C.) ethyl acetate. The crystalline ezatiostat hydrochloride ansolvate was dried at 30° C. for 48 hours, then cooled to room temperature and sieved. Analysis of the material by DSC and XRPD confirmed its identity as crystalline ezatiostat hydrochloride ansolvate, and Karl Fischer analysis showed a water content of 0.1%.Example 5Purifying Ezatiostat Hydrochloride Crystals to Form Pure Crystalline Ezatiostat Hydrochloride Ansolvate Form DCrude ezatiostat hydrochloride, 51.4 Kg, was added to a 600 L jacketed glass-lined reactor at room temperature, followed by 334 L of ethanol. The mixture was heated to 68° C. to completely dissolve the ezatiostat hydrochloride. The resulting solution was filtered into a 1300 L jacketed glass-lined reactor, and an additional 27 L ethanol warmed to 66° C. used to rinse the first reactor into the second reactor through the filter. The resulting solution in the second reactor was cooled to 63° C. and checked for complete dissolution; then 4 L of a seeding suspension of crystalline ezatiostat hydrochloride ansolvate in ethyl acetate was added, and the mixture cooled to 60° C. The remaining 4 L of the seeding suspension was added, and the mixture cooled to 47° C. over 2 hours. The solids in the mixture were shown by DSC to contain more than one form of ezatiostat hydrochloride, so the stages of heating to dissolution, cooling, and adding seeding suspension (this time 2×2 L), were repeated, then the mixture cooled to 41° C. This time the solids in the mixture were confirmed by DSC to be crystalline ezatiostat hydrochloride ansolvate. Ethyl acetate, 776 L, was added, and the mixture was cooled to 25° C. over 1.3 hours and further to 20° C. over an additional 5 hours, then cooled to −3° C. The mixture, containing crystalline ezatiostat hydrochloride ansolvate, was filtered and the solids washed twice with 54 L each of chilled (−5˜0)° C. ethyl acetate. The damp solids of crystalline ezatiostat hydrochloride ansolvate, 70 Kg, were dried in a vacuum oven at 25° C. for 16 hours, 35° C. for 7 hours, then at room temperature for 1 hour, then sieved. The crystalline ezatiostat hydrochloride ansolvate, 44.2 Kg, had a loss on drying at 40° C. under vacuum for 2 hours of 0.09%, and a water content by Karl Fischer analysis of 0.09%.
……………………………………………………………
U.S. Pat. No. 5,763,570
https://www.google.com/patents/US5763570
……………………………..
http://www.google.com/patents/WO2011156025A1?cl=en
Example 1. Preparation Of Ezatiostat Hydrochloride Ansolvate By Slurrying
[0082] Ezatiostat hydrochloride monohydrate was added to methyl tert-butyl ether at room temperature in excess, so that undissolved solids were present. The mixture was then agitated in a sealed vial at room temperature for 4 days, and the solids were then isolated by suction filtration. XRPD analysis of the solids established that the isolated solids were ezatiostat hydrochloride ansolvate.
[0083] Ezatiostat hydrochloride monohydrate was added to hexanes at 60 °C in excess, so that undissolved solids were present. The mixture was then agitated in a sealed vial at 60 °C for 4 days, and the solids were then isolated by suction filtration. XRPD analysis of the solids established that the isolated solids were ezatiostat hydrochloride ansolvate.
Example 2. Preparation Of Crystalline Ezatiostat Hydrochloride Ansolvate By Heating
[0084] DSC of crystalline ezatiostat hydrochloride monohydrate showed the pattern in FIG. 1, as discussed in paragraph above. Hot stage microscopy showed an initial melt followed by a recrystallization at 153 °C and a final melt at 166 °C. VT-XRPD, where XRPD patterns were obtained at 28 °C, 90 °C, and 160 °C during heating, and 28 °C after cooling of the formerly heated material, showed the presence of ezatiostat hydrochloride monohydrate at 28 °C and 90 °C during heating and of crystalline ezatiostat hydrochloride ansolvate at 160 °C and 28 °C after cooling of the formerly heated material. This confirmed that the transition at around 153/156 °C was a conversion of ezatiostat hydrochloride monohydrate form A to crystalline ezatiostat hydrochloride ansolvate form D and that the final DSC endothermic peak at about 177 °C (166 °C in the hot stage microscopy) was due to the melting of crystalline ezatiostat hydrochloride ansolvate. This was further confirmed by XRPD of the TG-IR material, where XRPD patterns obtained at room temperature both before and after heating to about 160 °C showed that the material before heating was form A and that the material after heating was form D ansolvate. DSC of crystalline ezatiostat hydrochloride ansolvate prepared by recrystallization showed the pattern in FIG. 5, with only the endothermic peak at about
177 °C followed by a broad endotherm at about (205 – 215) °C. Accordingly, the presence of the DSC endothermic peak at about 177 °C, for example at (177±2) °C, when measured under the conditions described above, is considered characteristic of crystalline ezatiostat hydrochloride ansolvate, and the substantial absence of thermal events at temperatures below this is considered indicative of the absence of other forms of ezatiostat hydrochloride
………………………………..
http://www.google.com/patents/WO2013082462A1?cl=en
Ezatiostat, also known as TLK199 or TER 199, is a compound of the formula:
[0003] Ezatiostat has been shown to induce the differentiation of HL-60 promyelocytic leukemia cells in vitro, to potentiate the activity of cytotoxic agents both in vitro and in vivo, and to stimulate colony formation of all three lineages of hematopoietic progenitor cells in normal human peripheral blood. In preclinical testing, ezatiostat has been shown to increase white blood cell production in normal animals, as well as in animals in which white blood cells were depleted by treatment with cisplatin or fluorouracil. Similar effects may provide a new approach to treating myelodysplasia syndrome (MDS).
[0004] Many conditions, including MDS, a form of pre-leukemia in which the bone marrow produces insufficient levels of one or more of the three major blood elements (white blood cells, red blood cells, and platelets), are characterized by depleted bone marrow.
Myelosuppression, which is characterized by a reduction in blood cell levels and in a reduction of new blood cell generation in the bone marrow, is also a common, toxic effect of many standard chemotherapeutic drugs.
[0005] Ezatiostat hydrochloride is the hydrochloride acid addition salt of ezatiostat.
Ezatiostat hydrochloride in a liposomal injectable formulation was studied in a clinical trial for the treatment of MDS, and results from this trial, reported by Raza et al., J. Hem. One, 2:20 (published online 13 May 2009), demonstrated that administration of TLK199 was well tolerated and resulted in multi-lineage hematologic improvement. Ezatiostat hydrochloride in a tablet formulation has been evaluated in a clinical trial for the treatment of MDS, as reported by Raza et al, Blood, 113:6533-6540 (prepublished online 27 April 2009) and a single-patient report by Quddus et al, J. Hem. One, 3:16 (published online 23 April 2010), and is currently being evaluated in clinical trials for the treatment of MDS and for severe chronic idiopathic neutropenia.
…………………………………..
http://www.google.com/patents/WO2013082462A1?cl=en
Example 1
[0048] 80 mg of crystalline ezatiostat hydrochloride was placed in a round bottom flask and dissolved in 25 mL of methanol. The solvent was then evaporated on a rotary evaporation apparatus under reduced pressure at 30 °C. After 30 minutes, the solid sample was removed from the round bottom flask and stored in a sealed vial at 2 °C in a refrigerator. Analysis of this sample was carried out within 24 hours of removing it from the rotary evaporation apparatus.
[0049] The resulting amorphous material was analyzed by 1H NMR, 13C NMR, DSC, and X-Ray powder diffraction experiments. The DSC conditions were 30 to 300 °C at 10 °C/ min using 7 mg of the amorphous material. The X-Ray powder diffraction was taken at 0-60 of 2theta. The crystalline ezatiostat hydrochloride was also analyzed.
Phase II trials: Ezatiostat is the first GSTP1-1 inhibitor shown to cause clinically significant and sustained reduction in RBC transfusions, transfusion independence, and multilineage responses in MDS patients. The tolerability and activity profile of ezatiostat may offer a new treatment option for patients with MDS. (source: Cancer. 2012 Apr 15;118(8):2138-47.)
|
References |
1: Galili N, Tamayo P, Botvinnik OB, Mesirov JP, Brooks MR, Brown G, Raza A. Prediction of response to therapy with ezatiostat in lower risk myelodysplastic syndrome. J Hematol Oncol. 2012 May 6;5:20. doi: 10.1186/1756-8722-5-20. PubMed PMID: 22559819; PubMed Central PMCID: PMC3407785.
2: Raza A, Galili N, Mulford D, Smith SE, Brown GL, Steensma DP, Lyons RM, Boccia R, Sekeres MA, Garcia-Manero G, Mesa RA. Phase 1 dose-ranging study of ezatiostat hydrochloride in combination with lenalidomide in patients with non-deletion (5q) low to intermediate-1 risk myelodysplastic syndrome (MDS). J Hematol Oncol. 2012 Apr 30;5:18. doi: 10.1186/1756-8722-5-18. PubMed PMID: 22546242; PubMed Central PMCID: PMC3416694.
3: Lyons RM, Wilks ST, Young S, Brown GL. Oral ezatiostat HCl (Telintra®, TLK199) and idiopathic chronic neutropenia (ICN): a case report of complete response of a patient with G-CSF resistant ICN following treatment with ezatiostat, a glutathione S-transferase P1-1 (GSTP1-1) inhibitor. J Hematol Oncol. 2011 Nov 2;4:43. doi: 10.1186/1756-8722-4-43. PubMed PMID: 22047626; PubMed Central PMCID: PMC3235963.
4: Raza A, Galili N, Smith SE, Godwin J, Boccia RV, Myint H, Mahadevan D, Mulford D, Rarick M, Brown GL, Schaar D, Faderl S, Komrokji RS, List AF, Sekeres M. A phase 2 randomized multicenter study of 2 extended dosing schedules of oral ezatiostat in low to intermediate-1 risk myelodysplastic syndrome. Cancer. 2012 Apr 15;118(8):2138-47. doi: 10.1002/cncr.26469. Epub 2011 Sep 1. PubMed PMID: 21887679.
5: Quddus F, Clima J, Seedham H, Sajjad G, Galili N, Raza A. Oral Ezatiostat HCl (TLK199) and Myelodysplastic syndrome: a case report of sustained hematologic response following an abbreviated exposure. J Hematol Oncol. 2010 Apr 23;3:16. doi: 10.1186/1756-8722-3-16. PubMed PMID: 20416051; PubMed Central PMCID: PMC2873355.
6: Steensma DP. Novel therapies for myelodysplastic syndromes. Hematol Oncol Clin North Am. 2010 Apr;24(2):423-41. doi: 10.1016/j.hoc.2010.02.010. Review. PubMed PMID: 20359635.
7: D’Alò F, Greco M, Criscuolo M, Voso MT. New treatments for myelodysplastic syndromes. Mediterr J Hematol Infect Dis. 2010 Aug 11;2(2):e2010021. doi: 10.4084/MJHID.2010.021. PubMed PMID: 21415972; PubMed Central PMCID: PMC3033133.
8: Raza A, Galili N, Callander N, Ochoa L, Piro L, Emanuel P, Williams S, Burris H 3rd, Faderl S, Estrov Z, Curtin P, Larson RA, Keck JG, Jones M, Meng L, Brown GL. Phase 1-2a multicenter dose-escalation study of ezatiostat hydrochloride liposomes for injection (Telintra, TLK199), a novel glutathione analog prodrug in patients with myelodysplastic syndrome. J Hematol Oncol. 2009 May 13;2:20. doi: 10.1186/1756-8722-2-20. PubMed PMID: 19439093; PubMed Central PMCID: PMC2694211.
9: Raza A, Galili N, Smith S, Godwin J, Lancet J, Melchert M, Jones M, Keck JG, Meng L, Brown GL, List A. Phase 1 multicenter dose-escalation study of ezatiostat hydrochloride (TLK199 tablets), a novel glutathione analog prodrug, in patients with myelodysplastic syndrome. Blood. 2009 Jun 25;113(26):6533-40. doi: 10.1182/blood-2009-01-176032. Epub 2009 Apr 27. PubMed PMID: 19398716.
| WO2013082462A1 * | Nov 30, 2012 | Jun 6, 2013 | Telik, Inc. | Amorphous ezatiostat ansolvate |
| US20120251496 * | Mar 20, 2012 | Oct 4, 2012 | Telik, Inc. | Ezatiostat for treating multiple myeloma |
……….
Known Yes1 kinase inhibitors, dasatinib and saracatinib.
| Compound name and NCGC ID | Structure | Clinical phase | Known targets | Yes1 IC50 (nM) |
|---|---|---|---|---|
| Dasatinib (1) NCGC00181129 |
 |
Approved | Lyn, PDGFR, KIT, Lck, BTK, Bcr–Abl, Fyn, Yes1, c-Src |
0.5 (<1.0)a |
| Saracatinib (2) NCGC00241099 |
 |
Phase II/III | c-Src, Bcr–Abl, Yes1, Lck | 6.2 (0.70)a |
| AEE-788 (3) NCGC00263149 |
 |
Phase I/II | EGFR, HER-2, VEGFR-2 | 17.5 (13.1)a |
| Dovitinib (4) NCGC00249685 |
 |
Phase III | FGFR, EGFR, PDGFR, VEGFR-1,2 | 31 (1.4)a |
| DCC-2036 (5) NCGC00263172 |
 |
Phase I/II | Bcr–Abl, Tie-2, Lyn, FLT3, VEGFR-2 | 2.5 (1.5)a |
| SGI-1776 (6) NCGC00263186 |
 |
Discontinued | Pim-1, FLT3 | 2670 (240)a |
| AMG-Tie-2-1 (7) NCGC00263199 |
 |
Preclinical | Tie-2 | 8.7 (22.0)a |
| AZ-23 (8) NCGC00250381 |
 |
Preclinical | Trk | 39.1 (3.0)a |
| Dorsomorphin (9) NCGC00165869 |
 |
Preclinical | AMPK, BMPR, TGFβ Receptor | 195.9 (29.8)a |
| AZ-628 (10) NCGC00250380 |
 |
Preclinical | Raf Kinase B,C | 348.3 (51.2)a |
- http://www.sciencedirect.com/science/article/pii/S0960894X13006677
- Data in parentheses were gathered by Reaction Biology Corp. using a [γ-33P]-ATP radiolabeled enzyme activity assay at an ATP concentration of 10 μM (www.reactionbiology.com).
Filed under: Phase3 drugs, Uncategorized Tagged: Ezatiostat, TELINTRA®, TLK-199, TLK199


















