-
![]()
![]() IDX 18719; IDX 719; Samatasvir
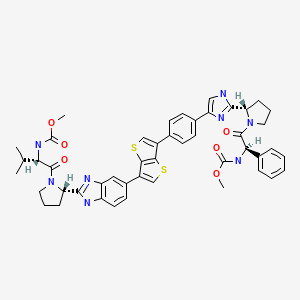
IDX 18719; IDX 719; SamatasvirN-((1R)-2-((2S)-2-(5-(4-(6-(2-((2S)-1-((2S)-2-((methoxycarbonyl)amino)-3-methylbutanoyl)pyrrolidin-2-yl)-3H-benzimidazol-5-yl)thieno(3,2-b)thiophen-3-yl)phenyl)-1H-imidazol-2-yl)pyrrolidin-1-yl)-2-oxo-1-phenylethyl)carbamate
Carbamic acid, N-((1R)-2-((2S)-2-(5-(4-(6-(2-((2S)-1-((2S)-2-((methoxycarbonyl)amino)-3-methyl-1-oxobutyl)-2-pyrrolidinyl)-1H-benzimidazol-6-yl)thieno(3,2-b)thien-3-yl)phenyl)-1H-imidazol-2-yl)-1-pyrrolidinyl)-2-oxo-1-phenylethyl)-, methyl ester
Carbamic acid, N-[(1R)-2-[(2S)-2-[5-[4-[6-[2-[(2S)-1-[(2S)-2-[(methoxycarbonyl)amino]-3-methyl-1-oxobutyl]-2-pyrrolidinyl]-1H-benzimidazol-6-yl]thieno[3,2-b]thien-3-yl]phenyl]-1H-imidazol-2-yl]-1-pyrrolidinyl]-2-oxo-1-phenylethyl]-, methyl ester
[(5)-l-((5)-2- {6-[5-(4- {(5)-2-[l-((R)-2-methoxycarbonylamino-2-phenyl- acetyl)-pyrrolidin-2-yl]-lH-imidazol-4-yl}-phenyl)-thieno[3,2-b]thiophen-2-yl)-lH- benzoimidazol-2-yl} -pyrrolidine- l-carbonyl)-2-methyl-propyl]-carbamic acid methyl ester
[(S)-1-((S)-2-{6-[6-(4-{(S)-2-[1-((R)-2-methoxycarbonylamino-2-phenyl-acetyl)-pyrrolidin-2-yl]-3H-imidazol-4-yl}-phenyl)-thieno[3,2-b]thiophen-3-yl]-1H-benzoimidazol-2-yl}-pyrrolidine-1-carbonyl)-2-methyl-propyl]-carbamic acid methyl ester
Carbamic acid, N-((1R)-2-((2S)-2-(5-(4-(6-(2-((2S)-1-((2S)-2-((methoxycarbonyl)amino)-3-methyl-1-oxobutyl)-2-pyrrolidinyl)-1H-benzimidazol-6-yl)thieno(3,2-b)thien-3-yl)phenyl)-1H-imidazol-2-yl)-1-pyrrolidinyl)-2-oxo-1-phenylethyl)-, methyl ester
Methyl N-((1R)-2-((2S)-2-(5-(4-(6-(2-((2S)-1-((2S)-2-((methoxycarbonyl)amino)-3-methylbutanoyl)pyrrolidin-2-yl)-3H-benzimidazol-5-yl)thieno(3,2-b)thiophen-3-yl)phenyl)-1H-imidazol-2-yl)pyrrolidin-1-yl)-2-oxo-1-phenylethyl)carbamate![]()
-
- Idenix Pharmaceuticals, Inc. phase 2
-
CAS Number: 1312547-19-5
-
C47-H48-N8-O6-S2
- 885.0782
- UNII-21P699C5FC
- deleted CAS Registry Numbers: 1458063-70-1
- INhttp://www.google.com/patents/WO2011075615A1?cl=en
![]()
![Figure US20120252721A1-20121004-C00517]() A 169 IN http://www.google.com/patents/US20120252721
A 169 IN http://www.google.com/patents/US20120252721![]()
![]() compd in
compd inhttp://www.google.com/patents/WO2014036244A1?cl=en
ANY ERROR amcrasto@gmail.com
samatasvir
Samatasvir is an orally-available pan-genotypic hepatitis C virus (HCV) non-structural protein 5A (NS5A) inhibitor in phase II clinical studies at Idenix for the treatment of treatment-naïve genotype 1-4 HCV-infected patients in combination with simeprevir and ribavirin.
![]() Jun 6, 2013
Jun 6, 2013Idenix Pharmaceuticals Announces Samatasvir (IDX719) Poster Presentations at the Asian Pacific Association for the Study of the Liver (APASL) Conference
CAMBRIDGE, Mass., June 6, 2013 (GLOBE NEWSWIRE) — Idenix Pharmaceuticals, Inc. (Nasdaq:IDIX), a biopharmaceutical company engaged in the discovery and development of drugs for the treatment of human viral diseases, today announced three poster presentations featuring clinical and preclinical data for samatasvir (IDX719), Idenix’s once-daily pan-genotypic NS5A inhibitor for the treatment of hepatitis C virus (HCV) infection, at the Asian Pacific Association for the Study of the Liver (APASL) Liver Week 2013, taking place in Singapore, June 6-10, 2013. Idenix recently initiated a phase II clinical trial (HELIX-1) evaluating an all-oral, direct-acting antiviral (DAA) HCV combination regimen of samatasvir and simeprevir (TMC435), a once-daily protease inhibitor jointly developed by Janssen R&D Ireland and Medivir AB.
The following abstracts will be presented in poster sessions during APASL Liver Week 2013 in the Conference Exhibition Hall on Friday, June 7, 2013, 8:30am – 5:30pm SGT:
- Abstract No. 2110: “Pharmacokinetics and Pharmacodynamics of IDX719, a Pan-Genotypic HCV NS5A Inhibitor, in Genotype 1, 2, 3 or 4 HCV-Infected Subjects.”
- Abstract No. 2121: “Hepatitis C Virus NS5A Inhibitor IDX719 Demonstrates Potent, Pan-genotypic Activity in Preclinical and Clinical Studies.”
- Abstract No. 2127: “IDX719, a Pan-genotypic HCV NS5A Replication Complex Inhibitor, Is a Promising Candidate for HCV Combination DAA Treatment.”
ABOUT SAMATASVIR (IDX719)
Samatasvir is an NS5A inhibitor with low picomolar, pan-genotypic antiviral activity in vitro. To date, samatasvir has been safe and well-tolerated after single and multiple doses of up to 150 mg in healthy volunteers for up to 14 days’ duration and up to 100 mg in HCV-infected patients up to 3 days’ duration. There have been no treatment-emergent serious adverse events reported in the program. Samatasvir has demonstrated potent pan-genotypic antiviral activity in HCV-infected patients with mean maximal viral load reductions up to approximately 4.0 log10 IU/mL across HCV genotypes 1-4 in a proof-of-concept, three-day monotherapy study.
The HELIX-1 trial is a 12-week, randomized, double-blind, parallel group study evaluating the safety and tolerability of samatasvir and simeprevir in addition to antiviral activity endpoints, with a target enrollment of 90 treatment-naïve, non-cirrhotic, genotype 1b or 4 HCV-infected patients. The HELIX-1 trial is the first study in HCV-infected patients to commence under a non-exclusive collaboration agreement signed with Janssen in January 2013. A second trial (HELIX-2) of samatasvir, simeprevir and TMC647055, a once-daily non-nucleoside polymerase inhibitor boosted with low-dose ritonavir being developed by Janssen, is expected to initiate in the second half of 2013.
ABOUT HEPATITIS C
Hepatitis C virus is a common blood-borne pathogen infecting three to four million people worldwide annually. The World Health Organization (WHO) estimates that more than 170 million people worldwide are chronically infected with HCV, representing a nearly 5-fold greater prevalence than human immunodeficiency virus.
ABOUT IDENIX
Idenix Pharmaceuticals, Inc., headquartered in Cambridge, Massachusetts, is a biopharmaceutical company engaged in the discovery and development of drugs for the treatment of human viral diseases. Idenix’s current focus is on the treatment of patients with hepatitis C virus (HCV) infection. For further information about Idenix, please refer to www.idenix.com.
-
![]()
- ………………………………………
![]()
- WO 2014036244
- http://www.google.com/patents/WO2014036244A1?cl=en
![Figure imgf000014_0001]()
-
[(5)-l-((5)-2-{6-[6-(4-{(5)-2-[l-((i?)-2- methoxycarbonylamino-2-phenyl-acetyl)-pyrrolidin-2-yl]-3H-imidazol-4-yl}-phenyl)- thieno[3,2-¾]thiophen-3-yl]- lH-benzoimidazol-2-yl} -pyrrolidine- 1 -carbonyl)-2-methyl- propyl]-carbamic acid methyl ester (“the Compound”), having the structure of Formula I:
(I) or an isotopic variant thereof, or a pharmaceutically acceptable salt or solvate thereof.
The Compound is a nonstructural protein 5A (NS5A) inhibitor. See U.S. Pat.App. Pub. Nos. US 2011/0150827 and US 2012/0252721, the disclosure of each of which is incorporated herein by reference in its entirety. The Compound is a potent and pan-genotypic inhibitor of HCV replication in vitro, with EC50 values ranging from 2 to 24 pM against HCV genotypes la, lb, 2a, 3a, 4a, and 5a. Id.
The Compound can be prepared according to the methods described in U.S.Pat. App. Pub. No. US 2011/0150827. The Compound can be also synthesized according to other methods apparent to those of skill in the art based upon the teaching herein.
![]()
- ………………
- WO 2011075615
- http://www.google.com/patents/WO2011075615A1?cl=en
- Example 36Synthesis of [(5)-l-((5)-2- {6-[5-(4- {(5)-2-[l-((R)-2-methoxycarbonylamino-2-phenyl- acetyl)-pyrrolidin-2-yl]-lH-imidazol-4-yl}-phenyl)-thieno[3,2-b]thiophen-2-yl)-lH- benzoimidazol-2-yl} -pyrrolidine- l-carbonyl)-2-methyl-propyl]-carbamic acid methyl esterA215
A215
1] Compound A215 was synthesized as shown
Scheme 27
[00612] Preparation of (S 2-[6-(5-bromo-thieno[3,2,b]thiophen-2-yl)-lH- benzoimidazol-2-yl] -pyrrolidine- 1-carboxylic acid tert-butyl ester E64. In a round bottom flask were added intermediate 66 (2.42 mmol) and 3,6-dibromo-thieno[3,2-b]thiophene (7.26 mmol). The system was purged and anhydrous dioxane (36 mL) was added. Then, NaHC(¾ 1M (7.26 mmol) and Pdl 18 (0.242 mmol) were added. The reaction mixture was stirred under reflux (110 °C) for 1.5 hrs. The reaction mixture was cooled down to room temperature and DCM was added. The mixture was washed with water and the organic layer dried, filtered, and concentrated under reduced pressure. The residue was purified by silica gel chromatography (eluent: DCM to DCM/MeOH 2%) to give intermediate E64 as a yellow foam in 19% yield. MS (ESI, EI+) m/z = 505.8 (MH+).[00613] Preparation of 6-(5-bromo-thieno[3,2,b]thiophen-2-yl)-(S -2-pyrrolidin-2-yl- lH-benzoimidazole, hydrochloride E65. Intermediate E65 was synthesized from
intermediate E64 (0.198 mmol), following the procedure as described for intermediate E47 (without purification) to give intermediate E65 as a yellow solid in quantitative yield. MS (ESI, EI+) m/z = 405.8 (MH+).
[00614] Preparation of ((5)-l- {(5)-2-[6-(5-bromo-thieno[3,2-b]thiophen-2-yl)-lH- benzoimidazol-2-yl]-pyrrolidine-l-carbonyl}-2-methyl-propyl)-carbamic acid methyl ester E66. Intermediate E65 (0.198 mmol) was dissolved in anhydrous DCM (5 mL). The intermediate 1 (0.198 mmol) was added, followed by HATU (0.257 mmol) and Et3N (0.792 mmol). The reaction mixture was stirred at room temperature for 45 min. DCM was added and the mixture was washed with water. The organic layer was dried over a2S04, filtered, and concentrated under reduced pressure. The residue was purified by silica gel
chromatography (eluent: DCM to DCM/MeOH 2%) to give intermediate E66 in quantitative yield. MS (ESI, EI+) m/z = 562.7 (MH+).
[00615] Preparation of (S 2-{4-[4-(5-{(5')-2-[l-((5,)-2-methoxycarbonylamino-3- methyl-butyryl)-pyrrolidin-2-yl]-3H-benzoimidazol-5-yl}-thieno[3,2-b]thiophen-2-yl)- phenyl]-lH-imidazol-2-yl}-pyrrolidine-l-carboxylic acid tert-butyl ester E67. Intermediate E67 was synthesized from intermediate E66 (0.196 mmol), following the procedure as described for the compound Al (1 10° C for 35 min). The residue was purified by silica gel chromatography (eluent: DCM to DCM/MeOH 4%) to give intermediate E67 as a yellow solid in 46% yield. MS (ESI, EI ) m/z = 794.2 (MH ).
[00616] Preparation of{2-methyl-(5)-l-[(5)-2-(6-{5-[4-((5)-2-pyrrolidin-2-yl-lH- imidazol-4-yl)-phenyl]-thieno[3,2-b]thiophen-2-yl}-lH-benzoimidazol-2-yl)-pyrrolidine-l- carbonyl]-propyl}-carbamic acid methyl ester, hydrochloride E68. Intermediate E68 was synthesized from intermediate E67 (0.086 mmol), following the procedure as described for intermediate E47 (without purification) to give intermediate E68 as an orange solid in quantitative yield. MS (ESI, EI+) m/z = 694.14 (MH+).
[00617] Preparation of [(5)-l-((5)-2- {6-[5-(4- {(5)-2-[l-((R)-2-methoxycarbonylamino- 2-phenyl-acetyl)-pyrrolidin-2-yl]-lH-imidazol-4-yl}-phenyl)-thieno[3,2-b]thiophen-2-yl)- lH-benzoimidazol-2-yl} -pyrrolidine- l-carbonyl)-2-methyl-propyl]-carbamic acid methyl ester A215. Compound A215 was synthesized from intermediate E68 (0.086 mmol) following the procedure as described for compound A114 to give compound A215 as a yellow solid in 48% yield. H NMR (DMS0-< 400 MHz) δ (ppm) 0.82 (d, J= 6.70 Hz, 3H), 0.86 (d, J= 6.70 Hz, 3H), 1.82-2.10 (m, 7H), 2.16-2.28 (m, 2H), 3.10-3.16 (m, 1H), 3.52-3.55 (m, 6H), 3.80-3.90 (m, 3H), 4.07 (t, J= 8.38 Hz, 1H), 5.04-5.19 (m, 2H), 5.37-5.53 (m, 1H), 6.91-7.1 (m, 1H), 7.30-7.88 (m, 15H), 11.77-1.95 (m, 1H), 12.29 (brs, 1H); MS (ESI, EI+) m/z = 885.3 (MH+).
- …………….
- WO 201213558
![shark]()
……………..
US 2013071352\
http://www.google.com/patents/US20130071352
Example 36 Synthesis of [(S)-1-((S)-2-{6-[5-(4-{(S)-2-[1-((R)-2-methoxycarbonylamino-2-phenyl-acetyl)-pyrrolidin-2-yl]-1H-imidazol-4-yl}-phenyl)-thieno[3,2-b]thiophen-2-yl)-1H-benzoimidazol-2-yl}-pyrrolidine-1-carbonyl)-2-methyl-propyl]-carbamic acid methyl ester A215
Compound A215 was synthesized as shown in 27.
Preparation of (S)-2-[6-(5-bromo-thieno[3,2,b]thiophen-2-yl)-1H-benzoimidazol-2-yl]-pyrrolidine-1-carboxylic acid tert-butyl ester E64. In a round bottom flask were added intermediate 66 (2.42 mmol) and 3,6-dibromo-thieno[3,2-b]thiophene (7.26 mmol). The system was purged and anhydrous dioxane (36 mL) was added. Then, NaHCO3 1M (7.26 mmol) and Pd118 (0.242 mmol) were added. The reaction mixture was stirred under reflux (110° C.) for 1.5 hrs. The reaction mixture was cooled down to room temperature and DCM was added. The mixture was washed with water and the organic layer dried, filtered, and concentrated under reduced pressure. The residue was purified by silica gel chromatography (eluent: DCM to DCM/MeOH 2%) to give intermediate E64 as a yellow foam in 19% yield. MS (ESI, EI+) m/z=505.8 (MH+).
Preparation of 6-(5-bromo-thieno[3,2,b]thiophen-2-yl)-(S)-2-pyrrolidin-2-yl-1H-benzoimidazole, hydrochloride E65. Intermediate E65 was synthesized from intermediate E64 (0.198 mmol), following the procedure as described for intermediate E47 (without purification) to give intermediate E65 as a yellow solid in quantitative yield. MS (ESI, EI+) m/z=405.8 (MH+).
Preparation of ((S)-1-{(S)-2-[6-(5-bromo-thieno[3,2-b]thiophen-2-yl)-1H-benzoimidazol-2-yl]-pyrrolidine-1-carbonyl}-2-methyl-propyl)-carbamic acid methyl ester E66. Intermediate E65 (0.198 mmol) was dissolved in anhydrous DCM (5 mL). The intermediate 1 (0.198 mmol) was added, followed by HATU (0.257 mmol) and Et3N (0.792 mmol). The reaction mixture was stirred at room temperature for 45 min. DCM was added and the mixture was washed with water. The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by silica gel chromatography (eluent: DCM to DCM/MeOH 2%) to give intermediate E66 in quantitative yield. MS (ESI, EI+) m/z=562.7 (MH+).
Preparation of (S)-2-{4-[4-(5-{(S)-2-[1-((S)-2-methoxycarbonylamino-3-methyl-butyryl)-pyrrolidin-2-yl]-3H-benzoimidazol-5-yl}-thieno[3,2-b]thiophen-2-yl)-phenyl]-1H-imidazol-2-yl}-pyrrolidine-1-carboxylic acid tert-butyl ester E67. Intermediate E67 was synthesized from intermediate E66 (0.196 mmol), following the procedure as described for the compound A1 (110° C. for 35 min). The residue was purified by silica gel chromatography (eluent: DCM to DCM/MeOH 4%) to give intermediate E67 as a yellow solid in 46% yield. MS (ESI, EI+) m/z=794.2 (MH+).
Preparation of{2-methyl-(S)-1-[(S)-2-(6-{5-[4-((S)-2-pyrrolidin-2-yl-1H-imidazol-4-yl)-phenyl]-thieno[3,2-b]thiophen-2-yl}-1H-benzoimidazol-2-yl)-pyrrolidine-1-carbonyl]-propyl}-carbamic acid methyl ester, hydrochloride E68. Intermediate E68 was synthesized from intermediate E67 (0.086 mmol), following the procedure as described for intermediate E47 (without purification) to give intermediate E68 as an orange solid in quantitative yield. MS (ESI, EI+) m/z=694.14 (MH+).
Preparation of [(S)-1-((S)-2-{6-[5-(4-{(S)-2-[1-((R)-2-methoxycarbonylamino-2-phenyl-acetyl)-pyrrolidin-2-yl]-1H-imidazol-4-yl}-phenyl)-thieno[3,2-b]thiophen-2-yl)-1H-benzoimidazol-2-yl}-pyrrolidine-1-carbonyl)-2-methyl-propyl]-carbamic acid methyl ester A215. Compound A215 was synthesized from intermediate E68 (0.086 mmol) following the procedure as described for compound A114 to give compound A215 as a yellow solid in 48% yield. 1H NMR (DMSO-d6, 400 MHz) δ (ppm) 0.82 (d, J=6.70 Hz, 3H), 0.86 (d, J=6.70 Hz, 3H), 1.82-2.10 (m, 7H), 2.16-2.28 (m, 2H), 3.10-3.16 (m, 1H), 3.52-3.55 (m, 6H), 3.80-3.90 (m, 3H), 4.07 (t, J=8.38 Hz, 1H), 5.04-5.19 (m, 2H), 5.37-5.53 (m, 1H), 6.91-7.1 (m, 1H), 7.30-7.88 (m, 15H), 11.77-1.95 (m, 1H), 12.29 (brs, 1H); MS (ESI, EI+) m/z=885.3 (MH+).
……………………..

US2012/252721
http://www.google.com/patents/US20120252721
Example 33Synthesis of [(S)-1-((S)-2-{6-[6-(4-{(S)-2-[1-((R)-2-methoxycarbonylamino-2-phenyl-acetyl)-pyrrolidin-2-yl]-3H-imidazol-4-yl}-phenyl)-thieno[3,2-b]thiophen-3-yl]-1H-benzoimidazol-2-yl}-pyrrolidine-1-carbonyl)-2-methyl-propyl]-carbamic acid methyl ester A169
Compound 169 was synthesized as shown in Scheme 24.
Preparation of (S)-2-{5-[4-(6-bromo-thieno[3,2-b]thiophen-3-yl)-phenyl]-1H-imidazol-2-yl}-pyrrolidine-1-carboxylic acid tert-butyl ester E78. To a mixture of DMF and water (20 mL/2.5 mL) were added Pd(PPh3)4 (0.1 mmol), 3,6-dibromo-thieno[3,2-b]thiophene (1.01 mmol), intermediate 6 (1.1 mmol), and sodium carbonate (4.04 mmol). The reaction mixture was degassed and irradiated for 1 hr at 80° C. Ethyl acetate was added and the organic layer was washed with water. The organic layer was dried over Na2SO4, filtered, and evaporated in vacuo. The residue was purified by silica gel chromatography (eluent: DCM-DCM/MeOH 98/2) to give intermediate E78 as a green gum in 41% yield. MS (ESI, EI+) m/z=532.19-530.31 (MH+).
Preparation of (S)-2-{5-[4-(6-{(S)-2-[1-((S)-2-methoxycarbonylamino-3-methyl-butyryl)-pyrrolidin-2-yl]-3H-benzoimidazol-5-yl}-thieno[3,2-b]thiophen-3-yl)-phenyl]-1H-imidazol-2-yl}-pyrrolidine-1-carboxylic acid tert-butyl ester E79. Compound 78 (0.198 mmol), intermediate 83 (0.228 mmol), and 1,1′-bis(di-tert-BP)ferrocene palladium dichloride (0.03 mmol) were added to a solution of dioxane (4 mL) and 1M NaHCO3 in water (0.594 mmol). The reaction mixture was irradiated at 90° C. for 1 hr. The mixture was diluted in dichloromethane and washed with water. The two layers were separated and the organic layer was concentrated under reduced pressure. The residue was purified by silica gel chromatography (eluent: DCM-DCM/MeOH 95/5) to give intermediate E79 as a brown foam in 70% yield. 1H NMR (CDCl3, 400 MHz) δ (ppm) 0.90-0.91 (m, 6H), 1.51 (s, 9H), 1.67-2.40 (m, 10H), 3.07-3.1 (m, 2H), 3.45-3.50 (m, 1H), 3.72 (s, 3H), 3.90 (m, 1H), 4.37 (m, 1H), 5.00-5.01 (m, 1H), 5.45-5.48 (m, 2H), 7.26-8.12 (m, 10H), 10.67 (m, 1H); MS (ESI, EI+) m/z=792.79 (MH−).
Preparation of [(S)-1-((S)-2-{6-[6-(4-{(S)-2-[1-((R)-2-methoxycarbonylamino-2-phenyl-acetyl)-pyrrolidin-2-yl]-3H-imidazol-4-yl}-phenyl)-thieno[3,2-b]thiophen-3-yl]-1H-benzoimidazol-2-yl}-pyrrolidine-1-carbonyl)-2-methyl-propyl]-carbamic acid methyl ester A169. Intermediate E79 (0.132 mmol) was dissolved in methanol (2.6 mL) and 4N HCl in dioxane (2.64 mL) was added. The mixture was stirred 1 hr at room temperature before concentration under reduced pressure. The residue was dissolved in DMF (2.6 mL) and the mixture was cooled down to −100C. TEA (0.924 mmol), intermediate 31 (0.139 mmol), and HATU (0.172 mmol) were added and the mixture was stirred at −100C for 1 hr. Ethyl acetate was added and the mixture was washed with water. The organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The residue was filtered on a SCX-2 column and purified by silica gel chromatography (eluent: DCM-DCM/MeOH 97/3) to give compound A169 as a beige solid in 74% yield.
1H NMR (CDCl3, 400 MHz) δ (ppm) 0.89-0.91 (m, 6H), 1.40-2.42 (m, 8H), 3.08-3.24 (m, 3H), 3.67 (m, 3H), 3.71 (m, 4H), 3.88-3.89 (m, 1H), 4.34-4.38 (m, 1H), 5.30-5.32 (m, 1H), 5.42-5.45 (m, 3H), 6.03-6.04 (m, 1H), 7.26-8.14 (m, 16H), 10.65 (m, 1H);
MS (ESI, EI+) m/z=885.8 (MH+).

| US20110150827 * | Dec 17, 2010 | Jun 23, 2011 | Idenix Pharmaceuticals, Inc. | 5,5-fused arylene or heteroarylene hepatitis c virus inhibitors |
| US20120252721 * | Mar 29, 2012 | Oct 4, 2012 | Idenix Pharmaceuticals, Inc. | Methods for treating drug-resistant hepatitis c virus infection with a 5,5-fused arylene or heteroarylene hepatitis c virus inhibitor |
Filed under: Phase2 drugs, Uncategorized Tagged: IDX 18719, IDX 719, Samatasvir







 samatasvir
samatasvir