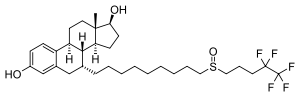
fulvestrant
| Fibrosis; Breast tumor; Female genital tract tumor; Uterus tumor |
Estrogen receptor antagonist
(7α,17β)-7-{9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl}estra-1,3,5(10)-triene-3,17-diol
129453-61-8 CAS
C32H47F5O3S
606.771
Fulvestrant is a drug treatment of hormone receptor-positive metastatic breast cancer in post-menopausal women with disease progression following anti-estrogen therapy. It is an estrogen receptor antagonist with no agonist effects, which works both by down-regulating and by degrading the estrogen receptor.
| Canada | 2351004 | 2003-02-18 | EXPIRY 2021-01-08 |
| United States | 6774122 | 2001-01-09 | EXPIRY 2021-01-09 |
Fulvestrant (Faslodex, AstraZeneca) is a drug treatment of hormone receptor-positive metastatic breast cancer in postmenopausal women with disease progression following anti-estrogen therapy. It is an estrogen receptor antagonist with no agonist effects, which works by down-regulating the estrogen receptor.[1] It is administered as a once-monthly injection.

Clinical uses
Fulvestrant is a selective estrogen receptor down-regulator (SERD). Fulvestrant is indicated for the treatment of hormone receptor positive metastatic breast cancer in postmenopausal women with disease progression following anti-estrogen therapy. The dosing schedule for fulvestrant remains under investigation in an attempt to optimize its effectiveness.[2]

Clinical trials
Metastatic or locally advanced breast cancer
Fulvestrant provided effective second-line therapy in this setting for postmenopausal women who had relapsed or progressed after previous endocrine therapy.[3]
In particular 4 clinical trials in this setting did show similar efficacy to the other hormonal agents (aromatase inhibitors and tamoxifen) with good tolerability profile. Fulvestrant had a lower incidence of joint disorders.[4][5]
NICE evaluation
The U.K. National Institute for Health and Clinical Excellence (NICE) said in 2011 that it found no evidence Faslodex was significantly better than existing treatments, so its widespread use would not be a good use of resources for the country’s National Health Service
The first month’s treatment of Faslodex, which starts with a loading dose, costs £1,044.82 ($1,666), and subsequent treatments cost £522.41 a month.
A month’s supply of anastrozole (Arimidex), which is off patent, costs £5.99, and letrozole (Femara) costs £84.86.[6][7][8]
Patent extension
The original patent for Faslodex expired in October 2004. Drugs subject to pre-marketing regulatory review are eligible for patent extension, and for this reason AstraZeneca got an extension of the patent to December 2011.[9][10]
AstraZeneca has filed later patents. There is no generic Faslodex available.[11] A later patent for Faslodex expires in January 2021.[12]
FASLODEX® (fulvestrant) injection for intramuscular administration is an estrogen receptor antagonist. The chemical name is 7-alpha-[9-(4,4,5,5,5-penta fluoropentylsulphinyl) nonyl]estra-1,3,5-(10)- triene-3,17beta-diol. The molecular formula is C32H47F5O3S and its structural formula is:
 |
Fulvestrant is a white powder with a molecular weight of 606.77. The solution for injection is a clear, colorless to yellow, viscous liquid.
Each injection contains as inactive ingredients: 10% w/v Alcohol, USP, 10% w/v Benzyl Alcohol, NF, and 15% w/v Benzyl Benzoate, USP, as co-solvents, and made up to 100% w/v with Castor Oil, USP as a co-solvent and release rate modifier.
-
Fulvestrant is a pure antiestrogen that represent a significant breakthrough in the treatment of breast cancer. Despite its pure antagonist activity, studies on ovariectomized rats have confirmed that fulvestrant, in contrast to Tamoxifen which acts like estrogen to reduce periosteal bone formation, does not alter estrogen-like or antiestrogenic effects. Fulvestrant also has some distinct advantages on target organs other than breast tissue.
-
Fulvestrant is a steroidal pure antiestrogen with a chemical structure similar to estradiol. Studies of etrogen receptor (ER) function have demonstrated that estradiol binding to the ER initiate a sequence of events. Fulvestrant antagonizes estrogen action by occupying the ER and preventing estrogen-stimulated gene activation, thus interfering with the estrogen related processes essential for cell-cycle competion.
-
WO Patent application No. 02/32922 describes a process for preparing an intermediate compound useful for preparing, e.g. fulvestrant, which process comprises aromatization of a compoud, and thereafter if necessary or desired, carrying out one or more of the following steps: (i) removing any hydroxy protecting group; (ii) converting a precursor group to a different such group.
-
EP Patent No. 0138504 relates to certain 7α-substituted derivatives of oestradiol and related steroids which possess antioestrogenic activity. US Patent No. 4659516 , EP Patent No. 0138504 and Bowler, Steroids 1989, 54, 71 describe a process for making steroids such as fulvestrant, by which 1,6-conjugate addition of an alkyl group to an estra-4,6-diene-3-one gave a ratio of 7α- to 7β-epimer of 1.2 : 1 (isolated). In WO 02/32922 it is stated that the ratio of epimers obtained using this process on an industrial scale is 1.9: 1.
-
US patent No 6288051 describes 7α-(5 -methylaminopentyl)-estratrienes.
-
There remains a need in the art for improved methods of preparing fluvestrant and other 7α-alkylated 19-norsteroids.
…………………
http://www.google.com/patents/EP1771462B1?cl=en



Preparative Example 16: Preparation of fulvestrant (Cp 9305) from Cp 9363 – indirect process (depicted in Figure 12)
-
A solution of 40.5 grams of Cp 9363 in 320 grams tetrahydrofuran and 81 grams methanol was cooled to 5°C and treated with a warm solution of 27 grams sodium (meta) periodate in 183 grams water. The mixture was allowed to stand at room temperature overnight, concentrated under vacuum and then dissolved in dichloromethane, extracted with water and evaporated to give 40 grams of Cp 9368 (fulvestrant 17-acetate).
-
The oily residue of Cp 9368 (40 grams) was dissolved in 320 grams of methanol under nitrogen and treated for 3 hours at room temperature with a solution of 20 grams of potassium hydroxide in 128 grams methanol. After neutralisation with 30 grams of acetic acid, the reaction mixture was concentrated under vacuum and then dissolved in dichloromethane, extracted with water and evaporated. The oily residue was crystallised from 400 grams of toluene, then dried under vacuum to constant weight. 26.6 grams of fulvestrant were obtained.
Example 17: Preparation of fulvestrant (Cp 9305) from Cp 9304 – direct process (depicted in Figure 9)
-
A solution of 41 grams of Cp 9304 in 328 grams tetrahydrofuran and 82 grams methanol was cooled to 5°C and treated with a warm solution of 27 grams sodium (meta)periodate in 185 grams water. The mixture was allowed to stand at room temperature overnight, concentrated under vacuum and then dissolved in dichloromethane, extracted with water, evaporated, and crystallised from toluene to give 28 grams of Cp 9305 (fulvestrant). Further purification can be effected by recrystallisation from ethyl acetate.

References
- S. Kansra, S. Yamagata, L. Sneade, L. Foster & N. Ben-Jonathan (2005). “Differential effects of estrogen receptor antagonists on pituitary lactotroph proliferation and prolactin release”. Mol Cell Endocrinol 239 (1-2): 27–36. doi:10.1016/j.mce.2005.04.008. PMID 15950373.
- Angela Mae Obermiller, PharmD; and Mehmet Sitki Copur, MD (2011). “The Longstanding Quest for a Better Endocrine Therapy Continues High-Dose Fulvestrant: Have We Found Its Effective Dose, Combination, Setting, or Sequence?”. Contemporary Oncology 3 (1).
- Croxtall, J. D.; McKeage, K. (2011). “Fulvestrant”. Drugs 71 (3): 363–380. doi:10.2165/11204810-000000000-00000. PMID 21319872.
- Fulvestrant in the treatment of advanced breast cancer: a systematic review and meta-analysis of randomized controlled trials. Valachis A, Mauri D, Polyzos NP, Mavroudis D, Georgoulias V, Casazza G. Crit Rev Oncol Hematol. 2010 Mar;73(3):220-7. Epub 2009 Apr 14. Review. PMID:19369092
- Fulvestrant for systemic therapy of locally advanced or metastatic breast cancer in postmenopausal women: a systematic review. Flemming J, Madarnas Y, Franek JA. Breast Cancer Res Treat. 2009 May;115(2):255-68. Epub 2008 Aug 6. Review. PMID:18683044
- UK cost body rules against AstraZeneca cancer drug, Reuters, Nov 9, 2011
- UK’s NICE says no to AstraZeneca breast cancer drug Faslodex, The Pharma Letter, 10 November 2011
- National Institute for Health and Clinical Excellence Guidance Breast cancer (metastatic) – fulvestrant
- Patent Term Extensions The United States Patent and Trademark Office.
- Determination of Regulatory Review Period for Purposes of Patent Extension; FASLODEX A Notice by the Food and Drug Administration on 04/17/2003
- Generic Faslodex Availability, Drugs.COM
- Pink Ribbon Blues: How Breast Cancer Culture Undermines Women’s Health By Gayle A. Sulik, Oxford University Press (Oct. 2010)
……………………….
VERY New patent
WO-2014064712
Process for the preparation of fulvestrant and its intermediates. Appears to be the first filing from Intas Pharmaceuticals on this API. Family members of the product patent, WO0151056 (assigned to AstraZeneca), expire in the EU states and in the US in 2021

Filed under: Uncategorized Tagged: fulvestrant

