cas no 1416314-55-0
C20 H18 F N5 O3
FYL-67 IS HYDROCHLORIDE
(S)-N-((3-(3-Fluoro-4-(4-(pyridin-2-yl)-1H-pyrazol-1-yl)phenyl)-2-oxo-oxazolidin-5-yl)methyl)acetamide
N-[[(5S)-3-[3-fluoro-4-[4-(2-pyridinyl)-1H-pyrazol-1-yl]phenyl]-2-oxo-5-oxazolidinyl]methyl]-Acetamide,
(S)-N-((3-(3-fluoro-4-(4-(pyridin-2-yl)-1H-pyrazol-1-yl) phenyl)-2-oxooxazolidin-5-yl)methyl)acetamide.
| Inventores | Youfu LUO, 罗有福, Zhenling WANG, 王震玲,Yuquan Wei, 魏于全 |
| Requerente | Si Chuan University, 四川大学 |
The discovery and application of antibiotics is one of the greatest achievements of mankind in the 20th century, the field of medicine, called a revolution of the history of the human fight against illness. Since then, the field of medicine into a bacterial disease caused by greatly reducing the golden age. Today, however, due to the widespread use of antibiotics or even abuse, the growing problem of bacterial resistance, humans are gradually approaching the “post-antibiotic era, the efficacy of antibiotics is gradually reduced. Clinical have been found on many new drug-resistant strains of methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), penicillin-resistant Streptococcus pneumoniae (PRSP) has seriously jeopardize the clinical treatment , the number of varieties of drugs less.
The compounds of the oxazolidinone linezolid was in the United States in 2000, mainly used in clinical acquired pneumonia, soft tissue infections, can also be used for the surgical treatment of infectious diseases, bones, lungs, cerebrospinal fluid permeability pharmacokinetic and tissue concentrations. Domestic and foreign the oxazolidinone drug development is a hot field
WO 2012171479
http://www.google.st/patents/WO2012171479A1?cl=en



The object compound (S N-{[3 - (3 - fluoro-4 - (4 - (2 - pyridyl) pyrazol-yl) phenyl) -2 - oxo-oxazol the embankment -5 - yl] methanone yl}
Weigh 150mg of the compound (26f), was dissolved with 10 ml of anhydrous THF was added under nitrogen protection, an ice water bath 154.1 mg t-BuOLi, ice-water bath after stirring for 5 minutes, 149.9 mg Compound 11, followed by ice-water bath was removed, go reaction at room temperature for 36 hours the reaction was stopped, by adding 10 mL of methylene chloride and 10 ml of water and 22μί acetic acid, stirred for 1 minute, the liquid separation, the aqueous phase was extracted with dichloromethane three times, the organic phases were combined, dried and purified by column chromatography to give the product ( 130 white solid 58 mg of yield of 38.2%.
1H-MR (400 MHz, CDC1 3): δ 8.61 (d, J = 4Hz, IH), 8.52 (d, J = 6.8Hz, 2.4H), 8.22 (s, IH), 7.94 (t, J = 8.8 Hz, IH), 7.77-7.69 (m, 2H), 7.55 (d, J = 8Hz, IH), 7.27-7.26 (m, IH), 7.18-7.15 (m, IH), 6.06 (t, J = 6Hz , IH), 4.86-4.80 (m, IH), 4.11 (t, J = 9.2Hz, IH), 3.86-3.82 (m, IH), 3.78-3.62 (m, 2H), 2.04 (s, 3H ;) .
13 C-MR (DMSO-e): δ 170.51, 154.47, 152.94, 151.26, 149.94, 139.70, 139.15, 137.43 129.96, 125.61, 125.19, 123.42, 122.19, 120.38, 114.52, 106.68, 72.29, 47.70, 41.84, 22.91.
ESI-MSm / z 418.08 (M + Na +).
………………….
Nanoscale (2013), 5(1), 275-283
Carrier-free nanoassemblies of a novel oxazolidinone compound FYL-67 display antimicrobial activity on methicillin-resistant Staphylococcus aureus
E-mail: luo_youfu@scu.edu.cn, wangzhenling2007@126.com;
Fax: +86-28-85164060 ;
Tel: +86-28-85164063
DOI: 10.1039/C2NR32505E
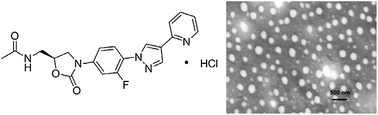
In this work, a novel oxazolidinone compound FYL-67 was synthesized, and the obtained FYL-67 could form nanoassemblies in aqueous solution by a self-assembly method without using any carrier, organic solvent, or surfactant. The prepared FYL-67 nanoassemblies had a particle size of 264.6 ± 4.3 nm. The FYL-67 nanoassemblies can be lyophilized into a powder form without any cryoprotector or excipient, and the re-dissolved FYL-67 nanoassemblies are stable and homogeneous. The in vitro release profile showed a significant difference between rapid release of free FYL-67 and much slower and sustained release of FYL-67 nanoassemblies. In vitro susceptibility tests were conducted in three strains of methicillin-susceptibleStaphylococcus aureus (MSSA) and three strains of methicillin-resistant Staphylococcus aureus(MRSA), using linezolid as a positive control. FYL-67 nanoassemblies exhibited excellent in vitro activity, with a minimum inhibitory concentration (MIC) value of 0.5 μg mL−1 against MRSA. In the in vitro post-antibiotic effect (PAE) evaluation, FYL-67 nanoassemblies showed a more powerful effect than linezolid. Besides, in vitro cytotoxicity tests indicated that FYL-67 nanoassemblies had a very low cytotoxicity on HEK293 cells and L02 cells. Furthermore, in both MSSA and MRSA systemic infection mouse models, FYL-67 nanoassemblies showed a lower ED50 than linezolid. In a murine model of MRSA systemic infection, FYL-67 nanoassemblies displayed an ED50 of less than 4.0 mg kg−1, which is 2.3-fold better than that oflinezolid. Our findings suggested that the FYL-67 nanoassemblies may be a potential drugcandidate in MRSA therapy.
 |
||
| Fig. 1 Synthetic route of the novel compound FYL-67. (i) 2-(pyridin-2-yl)malonaldehyde, p-TsOH (cat.),ethanol, reflux, 2 h; (ii) Fe, HCl, 95% ethanol, 1 h; (iii) Cbz–Cl, K2CO3, CH2Cl2, 2 h; (iv) (S)-1-acetamido-3-chloropropan-2-yl acetate, LiOt-Bu, THF, r.t.; (v) HCL (g), acetone, ethyl ether | ||
1H-NMR (400 MHz, CDCl3): δ 8.61 (d, J = 4 Hz, 1H), 8.52 (d, J = 6.8 Hz, 2.4H), 8.22 (s, 1H), 7.94 (t, J = 8.8 Hz, 1H), 7.77–7.69 (m, 2H), 7.55 (d, J = 8 Hz, 1H), 7.27–7.26 (m, 1H), 7.18–7.15 (m, 1H), 6.06 (t, J = 6 Hz, 1H), 4.86–4.80 (m, 1H), 4.11 (t, J = 9.2 Hz, 1H), 3.86–3.82 (m, 1H), 3.78–3.62 (m, 2H), 2.04 (s, 3H).
13C-NMR (DMSO-d6): δ 170.51, 154.47, 152.94, 151.26, 149.94, 139.70, 139.15, 137.43, 129.96, 125.61, 125.19, 123.42, 122.19, 120.38, 114.52, 106.68, 72.29, 47.70, 41.84, 22.91.
ESI-MS m/z418.08 (M + Na+).
1H-NMR (400 MHz, DMSO-d6) δ: 9.33 (s, 1H), 8.80 (s, 1H), 8.74 (d, J = 5.6 Hz, 1H), 8.45 (t, J = 7.2 Hz, 1H), 8.38–8.31 (m, 2H), 7.90 (t, J = 8.8 Hz, 1H), 7.81 (dd, J = 2.4 Hz, J = 16.4 Hz, 1H), 7.76 (t,J = 6.0 Hz, 1H); 7.55 (dd, J = 1.6 Hz, J = 8.8 Hz, 1H), 4.83–4.76 (m, 1H), 4.60 (br s, 1H), 4.20 (t, J = 8.8 Hz, 1H), 3.91–3.82 (m, 1H), 3.45 (t, J = 5.2 Hz, 2H), 1.85 (s, 3H);
13C-NMR (DMSO-d6) δ: 170.51, 154.47, 152.94, 151.26, 149.94, 139.70, 139.15, 137.43, 129.96, 125.61, 125.19, 123.42, 122.19, 120.38, 114.52, 106.68, 72.29, 47.70, 41.84, 22.91;
HR-MS(TOF) m/z calcd for C20H18FN5O3 [M + Cl−]: 430.1082, found: 430.1085; for C20H18FN5O3 [M + H+]: 396.1472, found: 396.1472.
PAPER

A concise, environmentally benign, and cost-effective route was developed for the large-scale preparation of 1, a novel oxazolidinone antibacterial candidate. The key intermediate 2-(1-(2-fluoro-4-nitrophenyl)-1H-pyrazol-4-yl)pyridine 7 was prepared with high purity by mild deamination of the regioisomeric mixture 21. The mixture was prepared from a nucleophilic SNAr reaction by selective C–N coupling of the secondary amine functionality of 4-(pyridin-2-yl)-1H-pyrazol-3-amine 14 with 1,2-difluoro-4-nitrobenzene 10 in optimized conditions with the primary amine group remaining intact. The gaseous nitrogen release rate and reaction mixture temperature of the deamination step can be well controlled by altering the feeding manner, thereby providing safety guarantees. The optimized synthetic strategy of 1 with an overall yield of 27.6%, including seven sequential transformations by only five solid–liquid isolations, significantly improved the product separation workup. The strategy bypassed time-consuming and laborious procedures for any intermediate involved as well as for the final API. This study presents a process enabling the rapid delivery of a multikilogram quantity of API with high purity.
\
(S)-N-((3-(3-Fluoro-4-(4-(pyridin-2-yl)-1H-pyrazol-1-yl)phenyl)-2-oxo-oxazolidin-5-yl)methyl)acetamide (1)
-
Brickner, S. J.; Hutchinson, D. K.; Barbachyn, M. R.; Manninen, P. R.; Ulanowicz, D. A.; Garmon, S. A.; Grega, K. C.; Hendges, S. K.; Toops, D. S.; Ford, C. W.; Zurenko, G. E.J. Med. Chem. 1996, 39, 673– 679
-
(a) Gong, C. Y.; Yang, T.; Yang, X. Y.; Liu, Y. Y.; Ang, W.; Tang, J. Y.; Pi, W. Y.; Xiong, L.; Chang, Y.; Ye, W. W.; Wang, Z. L.; Luo, Y. F.; Zhao, X.; Wei, Y. Q. Nanoscale. 2013, 5, 275–283(b) Luo, Y. F.; Wang, Z. L.; Wei, Y. Q.; Geng, F. WO/2012/171479,2012.
WO2008143649A2 * 4 Dez 2007 27 Nov 2008 Das Jagattaran Novel oxazolidinone compounds as antiinfective agents CN1172484A * 29 Jan 1996 4 Fev 1998 法玛西雅厄普约翰美国公司 Hetero-aromatic ring substituted phenyloxazolidinone antimicrobials
Filed under: Preclinical drugs, Uncategorized Tagged: FYL 67, Oxazolidinone, preclinical

