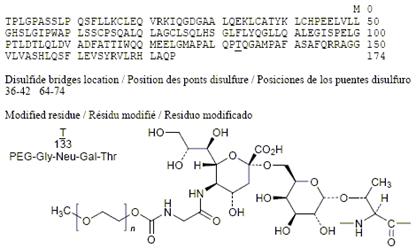
lipegfilgrastim
C 864 H 1369 N 225 O 258 S 9 [C 2 H 4 O] N
pegylated granulocyte colony stimulating factor; O3.133-[N5-(N-{[ω-methoxypoly (oxyethylene)] carbonyl} glycyl)-α-neuraminyl-(2 → 6)-α-D-galactopyranosyl]-L-methionyl -des-1-L-alanine-des-37-L-valine-des-38-L-serine-des-39-L-glutamic acid-human granulocyte colony-stimulating factor (G-CSF, pluripoietin)
Lonquex (lipegfilgrastim) has been approved to reduce the duration of neutropaenia (low white blood cell counts) and febrile neutropaenia in patients undergoing cytotoxic chemotherapy for cancer, and is given as a single subcutaneous dose per cycle of chemotherapy.
Like Neulasta (pegfilgrastim), Lonquex is a long-acting recombinant granulocyte colony-stimulating factor (G-CSF) and is dosed at the same frequency as Amgen’s drug.
http://www.pmlive.com/pharma_news/neulasta_rival_from_teva_cleared_in_eu_495953
Filed under: EU PIPELINE, EU SUBMISSION, Uncategorized Tagged: lipegfilgrastim
