Image may be NSFW.
Clik here to view.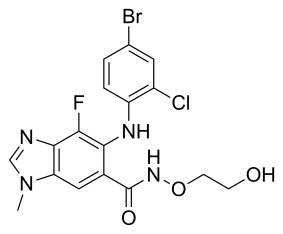
Selumetinib司美替尼
6-(4-bromo-2-chloroanilino)-7-fluoro-N-(2-hydroxyethoxy)-3-methylbenzimidazole-5-carboxamide
5-(4-Bromo-2-chlorophenylamino)-4-fluoro-1-methyl-1H-benzimidazole-6-carbohydroxamic acid 2-hydroxyethyl ester
6-(4-bromo-2-chloro- phenylamino)-7-fluoro-3 -methyl-3H-benzoimidazole-5-carboxylic acid (2-hydroxy- ethoxy)-amide
943332-08-9 (sulfate (1:1) salt) IS THE DRUG
Non-small-cell lung cancer (NSCLC) is the most common type of lung cancer. In October, AstraZeneca began a phase III trial of selumetinib in patients with KRAS mutation-positive NSCLC. AstraZeneca has also partnered with Roche Molecular Systems to develop a device to detect these mutations.
Selumetinib (AZD6244) is a drug being investigated for the treatment of various types of cancer, for example non-small cell lung cancer (NSCLC).
The gene BRAF is part of the MAPK/ERK pathway, a chain of proteins in cells that communicates input from growth factors. Activating mutations in the BRAF gene, primarily V600E (meaning that the amino acid valine in position 600 is replaced by glutamic acid), are associated with lower survival rates in patients with papillary thyroid cancer. Another type of mutation that leads to undue activation of this pathway occurs in the gene KRAS and is found in NSCLC. A possibility of reducing the activity of the MAPK/ERK pathway is to block the enzyme MAPK kinase (MEK), immediately downstream of BRAF, with the drug selumetinib. More specifically, selumetinib blocks the subtypes MEK1 and MEK2 of this enzyme.[1]
Selumetinib is a novel, selective, non-ATP-competitive inhibitor of MEK1/2 currently in phase III clinical development at AstraZeneca for the oral treatment of non-small lung cancer with KRAS mutation. Additional phase II trials are under way at both AstraZeneca and Array BioPharma for the treatment of other oncological indications, including colorectal cancer, thyroid cancer and malignant melanoma. AstraZeneca is conducting phase I/II clinical trials for the treatment of Kaposi’s sarcoma (AIDS-related) in combination with highly active anti-retroviral therapy (HAART). Also, phase I trials are ongoing at the companies targeting several solid tumors, including skin, pancreatic, colon, lung and breast tumors. The National Cancer Institute (NCI) is also evaluating selumetinib for the treatment of thyroid cancer, ovary cancer, myeloid leukemia, glioma, multiple myeloma, metastatic uveal melanoma, sarcoma, pancreatic cancer, plexiform neurofibromas and for the treatment of recurrent or persistent endometrial cancer. Additional early clinical trials are under way at the Massachusetts General Hospital for the treatment of cancers with BRAF mutations. No recent development has been reported for phase II clinical trials for the treatment of metastatic pancreatic cancer.
Image may be NSFW.
Clik here to view.
In addition to thyroid cancer, BRAF-activating mutations are prevalent in melanoma (up to 59%), colorectal cancer (5–22%), serousovarian cancer (up to 30%), and several other tumor types.[2]
KRAS mutations appear in 20 to 30% of NSCLC cases and about 40% of colorectal cancer.[1]
. The National Cancer Institute (NCI) is also evaluating selumetinib for the treatment of thyroid cancer, ovary cancer, myeloid leukemia, glioma, multiple myeloma, metastatic uveal melanoma, sarcoma, pancreatic cancer, plexiform neurofibromas and for the treatment of recurrent or persistent endometrial cancer. Additional early clinical trials are under way at the Massachusetts General Hospital for the treatment of cancers with BRAF mutations. No recent development has been reported for phase II clinical trials for the treatment of metastatic pancreatic cancer.
A Phase II clinical trial about selumetinib in NSCLC has been completed in September 2011;[3] one about cancers with BRAF mutations is ongoing as of June 2012.[4]
Selumetinib appears to efficiently target cancers with overactivation of MEK and associated cell signaling pathways. According to laboratory studies, selumetinib has an effect on human tumors at nanomolar concentrations. Potential advantages of selumetinib over marketed therapies include improved efficacy linked to a novel mechanism and ease of use based on the drug candidate’s oral formulation.
In 2013, AstraZeneca acquired exclusive worldwide rights to selumetinib from Array BioPharma.
AZD6244 (Selumetinib)
6-(4-Bromo-2- chloro-ρhenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid (2-hydroxy- ethoxy)-amide, or “Compound 1″, is exemplified in WO 03/077914 and possesses the following structural formula:
Clik here to view.

…………………………..
http://www.google.com/patents/US20030232869
Example 10
6-(4-Bromo-2-chloro-phenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid (2-hydroxy-ethoxy)-amide (29c)
Step A. 6-(4-Bromo-2-chloro-phenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid methyl ester 9a and 6-(4-Bromo-2-chloro-phenylamino)-7-fluoro-1-methyl-1H-benzoimidazole-5-carboxylic acid methyl ester
A solution of 6-(4-bromo-2-chloro-phenylamino)-7-fluoro-3H-benzoimidazole-5-carboxylic acid methyl ester 8b (150 mg, 0.38 mmol), iodomethane (28 μL, 0.45 mmol) and potassium carbonate (78 mg, 0.56 mmol) in dimethylformamide (1.5 mL) is stirred at 75° C. for one hour. The reaction mixture is diluted with ethyl acetate, washed with saturated aqueous potassium carbonate (2×), brine, and dried (Na2SO4). Flash column chromatography (20:1 methylene chloride/ethyl acetate) provides 56 mg (36%) of the more mobile 6-(4-bromo-2-chloro-phenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid methyl ester 9a as a white solid. 19F NMR (376 MHz, CD3OD)-133.5 (s). MS APCI (+) m/z 412, 414 (M+, Br pattern) detected. Also isolated is 54 mg (35%) of 6-(4-bromo-2-chloro-phenylamino)-7-fluoro-1-methyl-1H-benzoimidazole-5-carboxylic acid methyl ester as a white solid. 19F NMR (376 MHz, CD3OD)-139.9 (s). MS APCI (+) m/z 412, 414 (M+, Br pattern) detected.
Step B. 6-(4-Bromo-2-chloro-phenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid 10c
6-(4-Bromo-2-chloro-phenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid methyl ester 9a (56 mg, 0.14 mmol) is dissolved into 2:1 THF/water (3 mL) and NaOH (0.55 mL, 1.0 M aqueous solution, 0.55 mmol) is added. After stirring for two hours the reaction is reduced to one quarter initial volume via rotary evaporation and the remainder diluted to 50 mL with water. The aqueous solution is acidified to pH 2 by the addition of 1.0 M aqueous HCl and extracted with 1:1 tetrahydrofuran/ethyl acetate (3×), dried (Na2SO4) and concentrated under reduced pressure to provide 43 mg (79%) pure carboxylic acid as an off white solid. MS ESI (+) m/z 397, 398 (M+, Br pattern) detected.
Step C: 6-(4-Bromo-2-chloro-phenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid (2-vinyloxy-ethoxy)-amide 29a
6-(4-Bromo-2-chloro-phenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid 10c (2.00 g, 5.0 mmol), O-(2-vinyloxy-ethyl)-hydroxylamine (0.776 g, 7.5 mmol), HOBt (0.88 g, 6.5 mmol), triethylamine (1.61 mL, 2.3 mmol) and EDCI (1.3 g, 6.5 mmol) are dissolved in dimethylformamide (52 mL) and stirred at room temperature for 48 hours. The reaction mixture is diluted with ethyl acetate, washed with water (3×), saturated potassium carbonate (2×), saturated ammonium chloride (2×), brine, dried (Na2SO4) and concentrated under reduced pressure to an off-white solid. Trituration of the solid with diethyl ether provides 2.18 g (90%) desired product as an off-white solid. MS ESI (+) m/z 483, 485 (M+ Br pattern) detected.
Step D: 6-(4-Bromo-2-chloro-phenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid (2-hydroxy-ethoxy)-amide 29c
Hydrochloric acid (14 mL, 1.0 M aqueous solution, 14 mmol) is added to a suspension of 6-(4-bromo-2-chloro-phenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid (2-vinyloxy-ethoxy)-amide 29a (2.18 g, 4.50 mmol) in ethanol (50 mL) and the reaction mixture allowed to stir for 24 hours. The reaction mixture is concentrated to dryness by rotary evaporation and the solids partitioned between 3:1 ethyl acetate/tetrahydrofuran and saturated potassium carbonate. The aqueous phase is extracted with 3:1 ethyl acetate/tetrahydrofuran (3×), the combined organics dried (Na2SO4), and concentrated to provide 2.11 g (100%) 6-(4-bromo-2-chloro-phenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid (2-hydroxy-ethoxy)-amide as an off-white solid. MS ESI (+) m/z 457, 459 (M+, Br pattern) detected. 1H NMR (400 MHz, MeOH-d4) δ8.26 (s, 1H), 7.78 (s, 1H), 7.57 (d, 1H), 7.24 (dd, 1H), 6.40 (dd, 1H), 3.86 (s, 3H), 3.79 (m, 2H), 3.49 (m, 2H). 19F NMR (376 MHz, MeOH-d4)-133.68 (s).
…………
http://www.google.com/patents/WO2003077914A1?cl=en
Scheme 1
Scheme la
Scheme 2
Scheme 3
17 18
Scheme 4
25
Scheme 5
Example 1 and in this Example 9 by using the appropriate carboxylic acid and the appropriate hydroxylamine:
Example 10
6-(4-Bromo-2-chloro-phenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid (2-hydroxy-ethoxy)-amide (29c)Step A. 6-(4-Bromo-2-chloro-phenylamino)- 7-fluoro-3-methyl-3H-benzoimidazole-5- carboxylic acid methyl ester 9a and 6-(4-Bromo-2-chloro-phenylamino)-7-fluoro-l- methyl-lH-benzoimidazole-5-carboxylic acid methyl ester
A solution of 6-(4-bromo-2-chloro-phenylamino)-7-fluoro-3H-benzoimidazole-5-
carboxylic acid methyl ester 8b (150 mg, 0.38 mmol), iodomethane (28 μL, 0.45 mmol)
and potassium carbonate (78 mg, 0.56 mmol) in dimethylformamide (1.5 mL) is stirred at
75 °C for one hour. The reaction mixture is diluted with ethyl acetate, washed with saturated aqueous potassium carbonate (2x), brine, and dried (Na SO ). Flash column chromatography (20:1 methylene chloride/ethyl acetate) provides 56 mg (36%) of the
more mobile 6-(4-bromo-2-chloro-phenylamino)-7-fluoro-3 -methyl-3H-benzoimidazole-
5-carboxylic acid methyl ester 9a as a white solid. 19F NMR (376 MHz, CD3OD) -133.5
(s). MS APCI (+) m/z 412, 414 (M+, Br pattern) detected. Also isolated is 54 mg (35%)
of 6-(4-bromo-2-chloro-phenylamino)-7-fluoro-l-methyl-lH-benzoimidazole-5- carboxylic acid methyl ester as a white solid. 19F NMR (376 MHz, CD3OD) -139.9 (s).
MS APCI (+) m/z 412, 414 (M+, Br pattern) detected.
Step B. 6-(4-Bromo-2-chloro-phenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5- carboxylic acid 10c
6-(4-Bromo-2-chloro-phenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5- carboxylic acid methyl ester 9a (56 mg, 0.14 mmol) is dissolved into 2:1 THF/water (3 mL ) and NaOH (0.55 mL, 1.0 M aqueous solution, 0.55 mmol) is added. After stirring for two hours the reaction is reduced to one quarter initial volume via rotary evaporation and the remainder diluted to 50 mL with water. The aqueous solution is acidified to pH 2 by the addition of 1.0 M aqueous HCl and extracted with 1 : 1 tetrahydrofuran/ethyl acetate (3x), dried (Na2SO4) and concentrated under reduced pressure to provide 43 mg (79%) pure carboxylic acid as an off white solid. MS ESI (+) m/z 397, 398 (M+, Br pattern) detected.
Step C: 6-(4-Bromo-2-chloro-phenylamino)~ 7-fluoro-3-methyl-3H-benzoimidazole-5- carboxylic acid (2-vinyloxy-ethoxy)-amide 29a
6-(4-Bromo-2-chloro-phenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5- carboxylic acid 10c (2.00 g, 5.0 mmol), O-(2-vinyloxy-ethyl)-hydroxylamine (0.776 g, 7.5 mmol), HOBt (0.88 g, 6.5 mmol), triethylamine (1.61 mL, 2.3 mmol) and EDCI (1.3 g, 6.5 mmol) are dissolved in dimethylformamide (52 mL) and stirred at room temperature for 48 hours. The reaction mixture is diluted with ethyl acetate, washed with water (3x), saturated potassium carbonate (2x), saturated ammonium chloride (2x), brine, dried (Na2SO4) and concentrated under reduced pressure to an off-white solid. Trituration of the solid with diethyl ether provides 2.18 g (90%) desired product as an off- white solid. MS ESI (+) m/z 483, 485 (M+ Br pattern) detected.
Step D: 6-(4-Bromo-2-chloro-phenylamino)- 7-fluoro-3-methyl-3H-benzoimidazole-5- carboxylic acid (2-hydroxy-ethoxy) -amide 29c
Hydrochloric acid (14 mL, 1.0 M aqueous solution, 14 mmol) is added to a suspension of 6-(4-bromo-2-chloro-phenylamino)-7-fluoro-3 -methyl-3H-benzoimidazole -5-carboxylic acid (2-vinyloxy-ethoxy)-amide 29a (2.18 g, 4.50 mmol) in ethanol (50 mL) and the reaction mixture allowed to stir for 24 hours. The reaction mixture is concentrated to dryness by rotary evaporation and the solids partitioned between 3:1 ethyl acetate/tefrahydrofuran and saturated potassium carbonate. The aqueous phase is extracted with 3:1 ethyl acetate/tefrahydrofuran (3x), the combined organics dried (Na SO4), and concentrated to provide 2.11 g (100%) 6-(4-bromo-2-chloro- phenylamino)-7-fluoro-3 -methyl-3H-benzoimidazole-5-carboxylic acid (2-hydroxy- ethoxy)-amide as an off-white solid. MS ESI (+) m/z 457, 459 (M+, Br pattern) detected. 1H NMR (400 MHz, MeOH-c^) δ 8.26 (s, IH), 7.78 (s, IH), 7.57 (d, IH), 7.24 (dd, IH), 6.40 (dd, IH), 3.86 (s, 3H), 3.79 (m, 2H), 3.49 (m, 2H). 19F NMR (376 MHz, MeOH-d4) -133.68 (s).
………………
http://www.google.com/patents/EP1968948A2?cl=en
Example 1
Preparation of the Hydrogen sulfate salt of Compound 1
[0076] To a stirred suspension of 6-(4-bromo-2-chloro-phenylamino)-7-fiuoro-3- methyl-3H-benzoimidazole-5-carboxylic acid (2-hydroxy-ethoxy)-amide (100 g, 0.206 mol) (obtainable as described in Example 10 of WO 03/077914, which is incorporated herein by reference and as described below) in 2-butanone (680 mL) and water (115 mL) at 0-5 0C was added sulfuric acid (12.3 mL, 0.226 mol) followed by water (5 mL) maintaining a temperature of 10 °C or lower. The stirred mixture was heated to 65 0C and held for 30 minutes before filtering to remove any extraneous matter. The filter was washed with a mixture of 2-butanone (85 mL) and water (15 mL). The combined filtrates were heated to 72 0C before adding 2-butanone (500 mL) maintaining a temperature of between 60-72 0C. The resulting mixture was distilled at atmospheric pressure (approximate distillation temperature 73-74°C) until 500 mL of distillate had been collected.
[0077] A second aliquot of 2-butanone (500 mL) was added, maintaining the temperature of the mixture above 70 0C. The resulting mixture was distilled again until 250 mL of distillate had collected. The mixture was cooled to 0-5 0C over approximately 1 hour. The resulting slurry was filtered, washed with 2-butanone (240 mL) and dried under reduced pressure at 50 0C, until a constant weight was achieved, to give 6-(4-bromo-2-chloro- phenylamino)-7-fiuoro-3-methyl-3H-benzoimidazole-5-carboxylic acid (2-hydroxy-ethoxy)- amide hydrogen sulfate (103.5 g, 0.186 mol, 90% yield) as an off white crystalline solid.1H NMR (400 MHz, D6 DMSO) δ 3.58 (2H, t, CH2OH), 3.89 (2H, t, CH2ON), 3.99 (3H, s, CH3), 6.47 (IH, dd, ArH), 7.29 (IH, dd, ArH), 7.63 (IH, d, ArH), 7.91 (IH, s, ArH), 7.96 (3H, br, ROH, NH, SOH), 8.10 (IH, br, ArNH), 8.94 (IH, s, NCHN), 11.79 (IH, s, ONH). 13C NMR (100 MHz, D6 DMSO) δ 32.1 (CH3), 58.5 (CH2OH), 77.3 (CH2ON), 108.2 (CH), 109.6 (CBr), 115.8 (CH), 120.6 (CCl), 122.0 (C), 125.0 (CC=O), 129.4 (C), 130.5 (CH), 131.1 (CH), 132.3 (C), 140.6 (C), 145.8 (CF), 146.5 (CH), 164.2 (C=O). [0078] The results of the infrared analysis are shown in Figure 2. Spectral assignments axe summarized in Table 1.
Table 1
Wavenumber (cm“ ) Assignment 3,255 Includes the O-H stretching vibration of the primary alcohol group and the N-H stretching vibrations of the secondary aromatic amine and secondary amide groups.
3,200 – 2,700 Includes =C-H stretching vibrations of the aromatic ring and benzimidazole group and the aliphatic C-H stretching vibrations.
2,700 – 2,300 Includes the multiple NH+ stretching vibrations of the benzimidazole 1 : 1 sulfate salt group.
1,673 C=O stretching vibrations of the secondary amide group where
1,653 the carbonyl group is subject to different environmental effects such as hydrogen bonding.
1,640 – 1,370 Includes the C=C aromatic ring stretching vibrations, the C=C and C=N stretching vibrations of the benzimidazole group, the
O-H deformation vibration of the primary alcohol group and the aliphatic C-H deformation vibrations.
1,570 The CNH combination band of the secondary amide group.
1,506 Includes the CNH bending vibration of the secondary aromatic amine group.
1 ,213 The aryl C-F stretching vibration.
1,189 The asymmetric SO3 “ stretching vibration of the benzimidazole
1 : 1 sulfate salt group. 1,100 – 1,000 Includes the C-O stretching vibration of the primary alcohol group and the aryl C-Br stretching vibration. 1,011 The symmetric SO3 “ stretching vibration of the benzimidazole
1 :1 sulfate salt group. 920 – 600 Includes the C-H wag vibrations and C=C ring bending vibrations of the 1,2,4-trisubtituted aromatic ring and the benzimidazole group. 888 Includes the S-O(H) stretching vibration of the benzimidazole
1 : 1 sulfate salt group. Example IA
Preparation of the Hydrogen sulphate salt of Compound 1
[0079] Sulfuric acid (1.52 ml, 27.86 mmol) was added to a stirred suspension of 6-(4- bromo-2-chlorophenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid (2- hydroxyethoxy)-amide (1O g, 0.0214 mol) (obtainable as described in Example 10 of WO 03/077914, which is incorporated herein by reference and as described below) in tetrahydrofuran (THF) (62 ml) and water (8 ml) whilst maintaining a temperature of 10 0C or lower. The stirred mixture was heated to 65 0C and held for 30 minutes before filtering to remove any extraneous matter. THF (150 ml) was then added to the mixture maintaining the temperature above 60 0C. The mixture was then cooled to 0-5 0C over approximately 2 hour. The resulting slurry was filtered, washed with THF (30 ml) and dried under reduced pressure at 50 0C until a constant weight was achieved, to give 6-(4-bromo-2-chlorophenylamino)-7- fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid (2-hydroxyethoxy)-amide hydrogen sulfate (9.81g, 0.17 mol, 82% yield) as an off white crystalline solid. The material was the same as that produced in Example 1 above.
References
- Troiani, T.; Vecchione, L.; Martinelli, E.; Capasso, A.; Costantino, S.; Ciuffreda, L. P.; Morgillo, F.; Vitagliano, D.; d’Aiuto, E.; De Palma, R.; Tejpar, S.; Van Cutsem, E.; De Lorenzi, M.; Caraglia, M.; Berrino, L.; Ciardiello, F. (2012). “Intrinsic resistance to selumetinib, a selective inhibitor of MEK1/2, by cAMP-dependent protein kinase a activation in human lung and colorectal cancer cells”. British Journal of Cancer 106 (10): 1648–1659.doi:10.1038/bjc.2012.129. PMC 3349172. PMID 22569000.
- Davies, H.; Bignell, G. R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M. J.; Bottomley, W.; Davis, N.; Dicks, E.; Ewing, R.; Floyd, Y.; Gray, K.; Hall, S.; Hawes, R.; Hughes, J.; Kosmidou, V.; Menzies, A.; Mould, C.; Parker, A.; Stevens, C.; Watt, S.; Hooper, S.; Wilson, R.; Jayatilake, H.; Gusterson, B. A.; Cooper, C.; Shipley, J. (2002). “Mutations of the BRAF gene in human cancer”. Nature 417 (6892): 949–954. doi:10.1038/nature00766. PMID 12068308.
- Jump up^ ClinicalTrials.gov NCT00890825 Comparison of AZD6244 in Combination With Docetaxel Versus Docetaxel Alone in KRAS Mutation Positive Non Small Cell Lung Cancer (NSCLC) Patients
- Jump up^ ClinicalTrials.gov NCT00888134 AZD6244 in Cancers With BRAF Mutations
- Journal of the American Chemical Society, 2013 , vol. 135, 35 p. 12994 – 12997
-
8-1-2013Identification of potent Yes1 kinase inhibitors using a library screening approach.Bioorganic & medicinal chemistry letters
| WEDGE S R ET AL: “AZD2171: A HIGHLY POTENT, ORALLY BIOAVAILABLE, VASCULAR ENDOTHELIAL GROWTH FACTOR RECEPTOR-2 TYROSINE KINASE INHIBITOR FOR THE TREATMENT OF CANCER“, CANCER RESEARCH, AMERICAN ASSOCIATION FOR CANCER RESEARCH, US, vol. 65, no. 10, 15 May 2005 (2005-05-15), pages 4389-4400, XP008066714, ISSN: 0008-5472, DOI: 10.1158/0008-5472.CAN-04-4409 | ||
| 52 | * | WEDGE STEPHEN R ET AL: “ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration“, CANCER RESEARCH, AMERICAN ASSOCIATION FOR CANCER RESEARCH, US, vol. 62, no. 16, 15 August 2002 (2002-08-15), pages 4645-4655, XP002425560, ISSN: 0008-5472 |
| 53 | WEDGE, S.R. ET AL.: ‘ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration‘ CANCER RES vol. 62, 2002, pages 4645 – 4655 |
- Ho, Alan L.; Grewal, Ravinder K.; Leboeuf, Rebecca; Sherman, Eric J.; Pfister, David G.; Deandreis, Desiree; Pentlow, Keith S.; Zanzonico, Pat B. et al. (2013). “Selumetinib-Enhanced Radioiodine Uptake in Advanced Thyroid Cancer”. New England Journal of Medicine 368 (7): 623–32. doi:10.1056/NEJMoa1209288. PMC 3615415.PMID 23406027.
|
1-30-2009
|
TOSYLATE SALT OF 6- (4-BR0M0-2-CHL0R0PHENYLAMIN0) -7-FLUORO-N- (2-HYDROXYETHOXY) -3-METHYL-3H-BENZIMI DAZOLE- 5 – CARBOXAMIDE , MEK INHIBITOR USEFUL IN THE TREATMENT OF CANCER
|
|
|
9-17-2008
|
N3 alkylated benzimidazole derivatives as MEk inhibitors
|
|
|
6-27-2007
|
N3 alkylated benzimidazole derivatives as MEK inhibitors
|
|
|
12-19-2003
|
N3 alkylated benzimidazole derivatives as MEK inhibitors
|
|
6-6-2012
|
METHOD OF TREATMENT USING N3 ALKYLATED BENZIMIDAZOLE DERIVATIVES AS MEK INHIBITORS
|
|
|
6-6-2012
|
COMPOSITIONS COMPRISING N3 ALKYLATED BENZIMIDAZOLE DERIVATIVES AS MEK INHIBITORS AND METHODS OF USE THEREOF
|
|
|
5-16-2012
|
N3 ALKYLATED BENZIMIDAZOLE DERIVATIVES AS MEK INHIBITORS
|
|
|
8-24-2011
|
N3 ALKYLATED BENZIMIDAZOLE DERIVATIVES AS MEK INHIBITORS
|
|
|
7-6-2011
|
N3 ALKYLATED BENZIMIDAZOLE DERIVATIVES AS MEK INHIBITORS
|
|
|
11-31-2010
|
N3 ALKYLATED BENZIMIDAZOLE DERIVATIVES AS MEK INHIBITORS
|
|
|
8-18-2010
|
N3 ALKYLATED BENZIMIDAZOLE DERIVATIVES AS MEK INHIBITORS
|
|
|
5-28-2010
|
COMBINATION THERAPY COMPRISING AZD2171 AND AZD6244 OR MEK-INHIBITOR II
|
|
|
10-2-2009
|
PHARMACEUTICAL COMPOSITION 271
|
|
|
8-19-2009
|
N3 ALKYLATED BENZIMIDAZOLE DERIVATIVES AS MEK INHIBITORS
|
Filed under: cancer, Phase3 drugs Tagged: AZD6244, PHASE 3, Selumetinib Image may be NSFW.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.














