DW-116; fandofloxacin
164150-99-6 FREE BASE ,
164151-00-2., 164150-85-0
6-fluoro-1-(5-fluoropyridin-2-yl)-7-(4-methylpiperazin-1-yl)-4-oxoquinoline-3-carboxylic acid
6-Fluoro-1-(5-fluoropyridin-2-yl)-7-(4-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid
Dong Wha Pharmaceutical Co Ltd
Molecular Formula: C20H18F2N4O3
Molecular Weight: 400.378726
synthesis………….http://www.drugfuture.com/synth/syndata.aspx?ID=226498
http://www.google.com.mx/patents/WO1995005373A1
synthesis 1
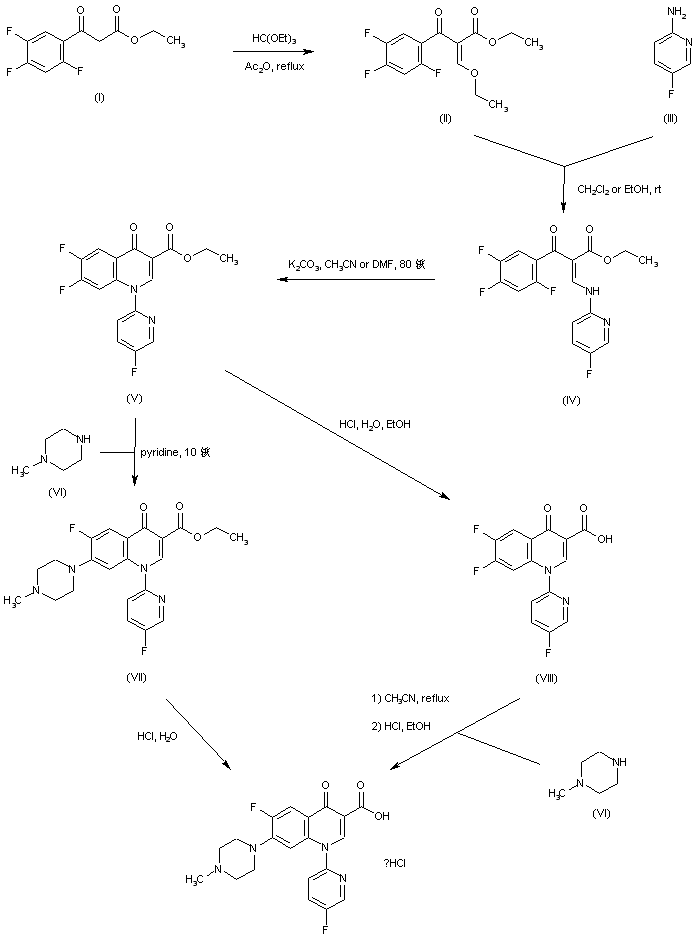
Condensation of ethyl 2,4,5-trifluorobenzoylacetate (I) with triethyl orthoformate in refluxing Ac2O produced the benzoyl ethoxyacrylate (II), which was further condensed with 2-amino-5-fluoropyridine (III) to afford enamine (IV). Cyclization of (IV) in the presence of K2CO3 gave rise to the quinolone (V). The 7-fluoride group of (V) was then displaced by N-methylpiperazine (VI) in cold pyridine to furnish the piperazinyl quinolone (VII). Finally, ester hydrolysis in (VII) under acidic conditions yielded the target compound. In a closely related procedure, ester (V) was hydrolyzed to acid (VIII) using HCl. Subsequent displacement of the 7-fluoride of (VIII) with N-methylpiperazine (VI) provided the desired piperazinyl quinolone.
synthesis 2

Condensation of ethyl 2,4,5-trifluorobenzoylacetate (I) with triethyl orthoformate in refluxing Ac2O produced the benzoyl ethoxyacrylate (II), which was further condensed with 2-amino-5-fluoropyridine (III) to afford enamine (IV). Cyclization of (IV) in the presence of K2CO3 gave rise to the quinolone (V). The 7-fluoride group of (V) was then displaced by N-methylpiperazine (VI) in cold pyridine to furnish the piperazinyl quinolone (VII). Finally, ester hydrolysis in (VII) under acidic conditions yielded the target compound. In a closely related procedure, ester (V) was hydrolyzed to acid (VIII) using HCl. Subsequent displacement of the 7-fluoride of (VIII) with N-methylpiperazine (VI) provided the desired piperazinyl quinolone.
Synthesis, pharmacokinetics, and biological activity of a series of new pyridonecarboxylic acid antibacterial agents bearing a 5-fluoro-2-pyridyl group or a 3-fluoro-4-pyridyl group at N-1
J Heterocycl Chem 1997, 34(3): 1021
|
6-31-2011
|
PHARMACEUTICAL COMPOSITION
|
|
|
8-24-2007
|
PHARMACEUTICAL COMPOSITION
|
|
|
6-29-2007
|
PHARMACEUTICAL COMPOSITION
|
|
|
10-28-2005
|
Identification and use of effectors and allosteric molecules for the alteration of gene expression
|
|
|
7-15-2005
|
Pharmaceutical composition
|
|
|
2-6-2004
|
Medicinal composition
|
|
|
4-20-2000
|
NOVEL QUINOLONE CARBOXYLIC ACID DERIVATIVES
|
|
|
3-6-1996
|
Quinolone carboxylic acid derivatives
|
|
|
2-24-1995
|
NOVEL QUINOLONE CARBOXYLIC ACID DERIVATIVES
|
Filed under: Phase2 drugs Tagged: fandofloxacin, phase 2

