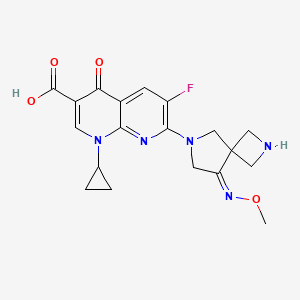
zabofloxacin, 219680-11-2
Example 1. l-Cyclopropyl-6-fluoro-7-[8-(methoxyimino)-2,6-diazaspiro[3,4]oct-6-yl]-4- oxo-l,4-dihydro[l,8]naphthyridine-3-carboxylic acid methanesulfonate
30 350mg of
7-[2-(t-buthoxycarbonyl)-8-(methoxyimino)-2,6-diazaspiro[3.4]oct-6-yl]-l- cyclopropyl-6-fluoro-4-oxo-l,4-dihydro[l,8]naphthyridine-3-carboxylic acid was dissolved in 5ml of dichloromethane and thereto 0.6ml of trifluoroacetic acid was dropped. The mixture was stirred for 5 hours at room temperature and thereto 10ml (if ethylether was added. It was stirred additionally for 1 hour and thus precipitated solid was filtered, dissolved in 5ml of diluted NaOH and neutralized with diluted hydrochloric acid. The precipitate thus obtained was filtered and dried. The resulting solid was added to 5ml of lN-methanesulfonic acid in ethanol and stirred for 1 hour. Thus obtained precipitate was filtered and dried to give 185g of the titled compound(yield : 47.8%). m.p. : 228- 229 °C
1H-NMR(DMSO-dG+CF3COOD, ppm): 0.97(s, 2H), 1.14(d, 2H), 2.48(s, 3H), 3.57(bs, IH), 3.88(s, 3H), 4.06-4.17(m, 411), 4.40(s, 2H), 4.49(s, 2H), 7.88(d, Hi, J=12.67Hz), 8.49(s, IH).
………………………………..
aspartate of 1-cyclopropyl-6-fluoro-7-(8-methoxyimino-2,6-diaza-spiro[3.4]oct-6-yl)-4-oxo-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid comprises a step of reacting 1-cyclopropyl-6-fluoro-7-(8-methoxyimino-2,6-diaza-spiro[3.4]oct-6-yl)-4-oxo-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid with aspartic acid in a solvent. The method can be represented by Scheme 1.
Example 1 Preparation of the D-Aspartic Acid Salt of 1-cyclopropyl-6-fluoro-7-(8-methoxyimino-2,6-diaza-spiro[3.4]oct-6-yl)-4-oxo-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid
1-Cyclopropyl-6-fluoro-7-(8-methoxyimino-2,6-diaza-spiro[3.4]oct-6-yl)-4-oxo-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid (5.0 g) was added to 50% ethanol (80 mL), and then the mixture was stirred at 50° C. for 10 minutes. D-Aspartic acid (2.0 g) was added and then the mixture was stirred at 50° C. for 1 hour. The mixture was cooled to room temperature, and then the resulting solid was collected by filtration. Ethanol (100 mL) was added to the filtrate, and then the mixture was stirred for 30 minutes. The resulting solid was collected by filtration to obtain a total of 5.55 g of the target compound (yield: 83%). Melting point: 200-201° C. 1H NMR (D2O): δ 0.97 (bs, 2H), 1.27 (d, 2H), 2.00 (dd, 1H, J=8.8, 17.6 Hz), 2.77 (dd, 1H, J=3.3, 17.0 Hz), 3.53 (bs, 1H), 3.84 (dd, 1H, J=3.3, 8.78 Hz), 4.01 (s, 3H), 4.31-4.45 (m, 8H), 7.46 (d, 1H, J=12.2 Hz), 8.42 (s, 1H).
Example 2 Preparation of L-Aspartic Acid Salt of 1-cyclopropyl-6-fluoro-7-(8-methoxyimino-2,6-diaza-spiro[3.4]oct-6-yl)-4-oxo-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid
1-Cyclopropyl-6-fluoro-7-(8-methoxyimino-2,6-diaza-spiro[3.4]oct-6-yl)-4-oxo-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid (500 mg) was added to 50% ethanol (20 mL), and then the mixture was stirred at 50° C. for 10 minutes. L-Aspartic acid (174 mg) was added and then the mixture was stirred at 50° C. for 1 hour. The mixture was cooled to room temperature. Ethanol (20 mL) was added to the reaction mixture, and then the mixture was stirred for 30 minutes. The resulting solid was collected by filtration to obtain 550 mg of the target compound (yield: 82%). Melting point: 205-206° C. 1H NMR (d6-DMSO): δ 0.93 (d, 2H, J=3.5 Hz), 1.20 (d, 2H, J=6.8 Hz), 2.42 (dd, 1H, J=9.2, 17.3 Hz), 2.59 (dd, 1H, J=3.3, 17.2 Hz), 3.50 (m, 1H), 3.59 (1H, dd, J=3.1, 9.1 Hz), 3.91 (s, 3H), 4.24 (m, 6H), 4.41 (br, 2H), 7.59 (d, 1H, J=12.4 Hz), 8.41 (s, 1H).
Example 3 Preparation of Hydrochloric Acid Salt, Phosphate Salt, and Formate Salt of 1-cyclopropyl-6-fluoro-7-(8-methoxyimino-2,6-diaza-spiro[3.4]oct-6-yl)-4-oxo-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid
3-1 Hydrochloric Acid Salt
Ethanol (3 mL) was cooled to 0° C. and acetyl chloride (1.13 mL) was added, and then the mixture was stirred for 30 minutes. 1-Cyclopropyl-6-fluoro-7-(8-methoxyimino-2,6-diaza-spiro[3.4]oct-6-yl)-4-oxo-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid (800 mg) was added to the reaction mixture, and then stirred at 0° C. for 30 minutes. Tetrahydrofuran (4 mL) was added, and then the mixture was stirred for 30 minutes. The resulting solid was collected by filtration and dried to obtain 776 mg of the target compound (yield: 89%). Melting point: 244-245° C. 1H NMR (d6-DMSO): δ 1.07 (d, 2H, J=4.7 Hz), 1.21 (d, 2H, J=6.8 Hz), 3.68 (m, 1H), 3.94 (s, 3H), 4.17 (m, 2H), 4.40 (s, 2H), 4.53 (s, 2H), 8.03 (d, 1H, J=12.5 Hz), 8.59 (s, 1H).
Han J, Kim JC, Chung MK, Kim B, Choi DR.
Biol Pharm Bull. 2003 Jun;26(6):832-9
Jin HE, Kang IH, Shim CK.
J Pharm Pharm Sci. 2011;14(3):291-305.
Jin HE, Lee KR, Kang IH, Chung SJ, Shim CK.
J Pharm Biomed Anal. 2011 Mar 25;54(4):873-7. doi: 10.1016/j.jpba.2010.11.001. Epub 2010 Nov 9.
Kosowska-Shick, K.; Credito, K.; Pankuch, G.A.; Lin, G.; Bozdogan, B.; McGhee, P.; Dewasse, B.;
Choi, D.-R.; Ryu, J.M.; Appelbaum, P.C. Antipneumococcal activity of DW-224a, a new
quinolone, compared to those of eight other agents. Antimicrob. Agents Chemother. 2006, 50,
2064–2071.
Park, H.-S.; Kim, H.-J.; Seol, M.-J.; Choi, D.-R.; Choi, E.-C.; Kwak, J.-H. In vitro and in vivo
antibacterial activities of DW-224a, a new fluoronaphthyridone. Antimicrob. Agents Chemother.
2006, 50, 2261–2264.
Dong Wha Pharmaceutical Co. Ltd. A study to evaluate efficacy and safety profile of
Zabofloxacin tablet 400 mg and moxifloxacin tablet 400 mg. Available online:
http://www.clinicaltrials.gov/ct2/show/NCT01658020 (accessed on 15 July 2013).
Dong Wha Pharmaceutical Co. Ltd. A new quinolone antibiotic. Available online:
http://www.dong-wha.co.kr/english/rnd/rnd02_03.asp (accessed on 15 April 2013).
| US4957922 * | Mar 29, 1989 | Sep 18, 1990 | Bayer Aktiengesellschaft | Infusion solutions of 1-cyclopropyl-6-fluoro-1,4-di-hydro-4-oxo-7-(1-piperazinyl)-quinoline-3-carboxylic acid |
| US5563149 * | Aug 8, 1994 | Oct 8, 1996 | Cheil Foods & Chemicals, Inc. | Aqueous solutions of pyridone carboxylic acids |
| US6552196 * | Sep 6, 2001 | Apr 22, 2003 | Dong Wha Pharmaceutical Industrial Co., Ltd. | Quinolone carboxylic acid derivatives |
|
7-23-2010
|
ASPARTATE OF 1-CYCLOPROPYL-6-FLUORO-7-(8-METHOXYIMINO-2,6-DIAZA-SPIRO[3.4]OCT-6-YL)-4-OXO-1,4-DIHYDRO-[1,8]NAPHTHYRIDINE-3-CARBOXYLIC ACID, METHOD FOR PREPARING THE SAME, AND ANTIMICROBIAL PHARMACEUTICAL COMPOSITION COMPRISING THE SAME
|
Filed under: Phase3 drugs, Uncategorized Tagged: zabofloxacin