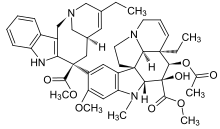

4-(acetyloxy)- 6,7-didehydro- 15-((2R,6R,8S)-4-ethyl- 1,3,6,7,8,9-hexahydro- 8-(methoxycarbonyl)- 2,6-methano- 2H-azecino(4,3-b)indol-8-yl)- 3-hydroxy- 16-methoxy- 1-methyl- methyl ester,
71486-22-1 cas
(2R,3R)-2,3-Dihydroxysuccinic acid – methyl (2ξ,3β,4β,5α,12β,19α)-4-acetoxy-15-[(12S,14R)-16-ethyl-12-(methoxycarbonyl)-1,10-diazatetracyclo[12.3.1.03,11.04,9]octadeca-3(11),4,6, 8,15-pentaen-12-yl]-3-hydroxy-16-methoxy-1-methyl-6,7-didehydroaspidospermidine-3-carboxylate (2:1)
Vinorelbine (trade name Navelbine) is an anti-mitotic chemotherapy drug that is given as a treatment for some types of cancer, including breast cancer and non-small cell lung cancer.
Clinicians sometimes use the abbreviation “NVB” for vinorelbine, although (like many medical abbreviations) it is not a unique identifier.
The antitumor activity is due to inhibition of mitosis through interaction with tubulin.[2] Vinorelbine is the first 5´NOR semi-synthetic vinca alkaloid. It is obtained by semi-synthesis from alkaloids extracted from the rosy periwinkle, Catharanthus roseus. It is marketed in India by Abbott Healthcare under the brand name Navelbine.
History
Vinorelbine was invented by the pharmacist Pierre Potier and his team from the CNRS in France in the 1980s and was licensed to the oncology department of the Pierre Fabre Group. The drug was approved in France in 1989 under the brand name Navelbine for the treatment of non-small celllung cancer. It gained approval to treat metastatic breast cancer in 1991. Vinorelbine received approval by the United States Food and Drug Administration (FDA) in December 1994 sponsored by Burroughs Wellcome Company. Pierre Fabre Group now markets Navelbine in the U.S., where the drug went generic in February 2003.
In most European countries, vinorelbine is approved to treat non-small cell lung cancer and breast cancer. In the United States it is approved only for non-small cell lung cancer.
NAVELBINE (vinorelbine tartrate) Injection is for intravenous administration. Each vial contains vinorelbine tartrate equivalent to 10 mg (1-mL vial) or 50 mg (5-mL vial) vinorelbine in Water for Injection. No preservatives or other additives are present. The aqueous solution is sterile and nonpyrogenic. Vinorelbine tartrate is a semi-synthetic vinca alkaloid with antitumor activity. The chemical name is 3′,4′-didehydro-4′-deoxy-C’-norvincaleukoblastine [R-(R*,R*)-2, 3-dihydroxybutanedioate (1:2)(salt)]. Vinorelbine tartrate has the following structure:
vinorelbine tartrate is a white to yellow or light brown amorphous powder with the molecular formula C45H54N4O8•2C4H6O6 and molecular weight of 1079.12. The aqueous solubility is > 1,000 mg/mL in distilled water. The pH of NAVELBINE (vinorelbine tartrate) Injection is approximately 3.5.
Uses
As stated above, Vinorelbine is approved for the treatment of non small cell lung cancer and metastatic breast cancer. It is also active inrhabdomyosarcoma.[3]
Oral formulation
An oral formulation has been marketed and registered in most European countries for the same settings. It has similar efficacy as the intravenous formulation, avoids venous toxicities of an infusion and is easier to take.

Side effects
Vinorelbine has a number of side-effects that can limit its use:
Chemotherapy-induced peripheral neuropathy (a progressive, enduring and often irreversible tingling numbness, intense pain, and hypersensitivity to cold, beginning in the hands and feet and sometimes involving the arms and legs[4]), lowered resistance to infection, bruising or bleeding, anaemia,constipation, diarrhea, nausea, tiredness and a general feeling of weakness (asthenia), inflammation of the vein into which it was injected (phlebitis). Seldom severe hyponatremia is seen.
Less common effects are hair loss and allergic reaction.
http://www.google.com/patents/EP2135872A1?cl=en


References
- Marty M, Fumoleau P, Adenis A, Rousseau Y, Merrouche Y, Robinet G, Senac I, Puozzo C (2001). “Oral vinorelbine pharmacokinetics and absolute bioavailability study in patients with solid tumors”. Ann Oncol 12 (11): 1643–9. doi:10.1023/A:1013180903805. PMID 11822766.
- Jordan, M.A.; Wilson, L. (2004). “Microtubules as a target for anticancer drugs.”. Nature Reviews. Cancer 4 (4): 253–65. doi:10.1038/nrc1317.PMID 15057285.
- Casanova, M; Ferrari, A; Spreafico, F; Terenziani, M; Massimino, M; Luksch, R; Cefalo, G; Polastri, D et al. (2002). “Vinorelbine in previously treated advanced childhood sarcomas: Evidence of activity in rhabdomyosarcoma”. Cancer 94 (12): 3263–8. doi:10.1002/cncr.10600. PMID 12115359.
- del Pino BM. Chemotherapy-induced Peripheral Neuropathy. NCI Cancer Bulletin. Feb 23, 2010;7(4):6.
Filed under: GENERIC DRUG Tagged: Vinorelbine
