-
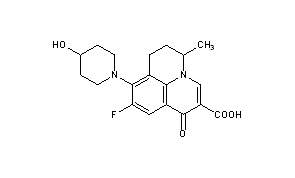
(+-)-9-Fluoro-6,7-dihydro-8-(4-hydroxypiperidino)-5-methyl-1-oxo-1H,5H-benzo(ij)quinolizine-2-carboxylic acid
- CCRIS 4066
- Jinofloxacin
- Nadifloxacin
- Nadifloxacine
- Nadifloxacine [INN-French]
- Nadifloxacino
- Nadifloxacino [INN-Spanish]
- Nadifloxacinum
- Nadifloxacinum [INN-Latin]
- Nadixa
- OPC-7251
- S-Nadifloxacin
- UNII-6CL9Y5YZEQ
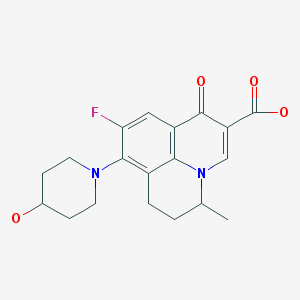
Nadifloxacin is chemically, 9-fluoro-6,7-dihydro-8-(4-hydroxy-l-pyperidinyl)-5-methyl- l-oxo-lH,5H-benzo(I,j)quinolizine-2-carboxylic acid of Formula I provided below.
FORMULA I Nadifloxacin is a synthetic quinolone with potent broad-spectrum anti-bacterial activity. Nadifloxacin inhibits the enzyme DNA gyrase that is involved in bacterial DNA synthesis and replication, thus inhibiting the bacterial multiplication. RS-nadifloxacin and S-nadifloxacin, in particular, exhibit strong antibacterial activity against Gram-positive, Gram-negative and anaerobic bacteria, resistant Gram-positive organisms such as methicillin-resistant Staphylococcus aureus (MRSA), quinolone-resistant Staphylococcus aureus, coagulase negative staphylococci, such as methicillin-resistant Staphylococcus epidermidis (MRSE), enterococci, betahemolytic streptococci and viridans group of streptococci, mycobacteria and newly emerging nosocomial pathogens such as Chryseobacterium meninges epticum, and Gram-negative pathogens such as E.coli, Klebsiella, Proteus, Serratia, Citrobacter and Pseudomonas. Recently, it has also been shown that S-(-)-nadifloxacin, in particular exhibits potent antibacterial activity against glycopeptide intermediate S. aureus (GISA), vancomycin intermediate S. aureus (VISA) and vancomycin-resistant S. aureus (VRSA). Nadifloxacin is also active against quinolone-resistant Staphylococci.
Nadifloxacin is marketed in the form of cream for topical application for the treatment of acne vulgaris, folliculitis and sycosis vulgaris. It is also indicated for the treatment of topical bacterial infections with susceptible bacteria.
The use of quinolone antibiotics to treat infections is known art in the field of ophthalmic pharmaceutical compositions and methods of treatment. Several quinolone antibacterial agents available in the market include gatifloxacin (available as Zymar®), Levofloxacin (available as Quixin® or Iquix®), Ciprofloxacin (available as Ciloxan®), Ofloxacin (available as Ocuflox®), Lomefloxacin (available as Lomeflox®), Moxifloxacin (available as Vigamox®) and Norfloxacin (available as Chibroxin®).
U.S. Patent No. 4,844,902 discloses a topically applicable formulation comprising by weight about 0.01 to 30% of an anti-bacterially active compound, 0.01 to 10% of a corticosteroid and a carrier. U.S. Patent No. 6,333,045 discloses liquid pharmaceutical compositions of gatifloxacin or salt thereof and disodium edetate.
U.S. Patent No. 6,716,830 discloses ophthalmic dosage forms of moxifioxacin or salts thereof in a concentration of 0.1% to 1% (w/w) and pharmaceutically acceptable vehicle.
U.S. Patent No. 6,359,016 relates to topical suspension formulations containing ciprofloxacin and dexamethasone.
U.S. Patent No 4,399,134 discloses processes for the preparation of nadifloxacin or salts thereof and antibacterially effective pharmaceutical compositions of nadifloxacin. Typical dosage forms include tablets, pills, powders, liquid preparations, suspensions, emulsions, granules, capsules, suppositories, and injectable preparations (solutions, suspensions, etc).
U.S. Patent No 6,884,768 discloses solid oral pharmaceutical compositions that includes nadifloxacin, an absorbefacient and taurine compounds.
U.S. Patent Application 20060183698 describes topical ophthalmic formulation that includes serum electrolytes; an antimicrobial compound and an anti-inflammatory or steroidal compound. Several antimicrobial agents have been disclosed including nadifloxacin.
U.S. Patent Application 20040176337 discloses topical . compositions of benzoquinolizine-2-carboxylic acid antimicrobial drug.
U.S. Patent Application 20040176321 discloses injectable pharmaceutical composition for intravenous delivery of an active agent that includes RS-(±)-nadifloxacin; S-(-)- nadifloxacin and hydrates thereof; or S~(-)-nadifloxacin arginine and salts thereof. PCT Publication WO 04/00360 describes pharmaceutical compositions of several active ingredients including nadifloxacin for topical use for treatment of dermatosis.
European Patent EP 275,515 and U.S. Patent No. 4,923,862 disclose aqueous pharmaceutical compositions of levofloxacin and ofloxacin or salts thereof.
PCT application WO 02/39993 discloses a stable pharmaceutical preparation of a combination drug, comprising an anti-infective agent, selected from the group consisting of quinolone derivatives, amino-glycoside derivatives and their pharmaceutically acceptable salts; an ant-inflammatory agent which is a corticosteroid; a complexation enhancing polymer; a solubilizer exhibiting an inclusion phenomena; pharmaceutically acceptable excipients within a suitable carrier system.
Journal of Ocular Pharmacology and Therapeutics, vol 23(3): 243-256, 2007 discloses (7- [(3R)-3 -aminohexahydro- 1 H-azepine- 1 -yl]-8-chloro- 1 -cyclopropyl-6-fluoro- 1 ,4-dihydro- 4-oxo-3-quinolinecarboxylivc acid as the topical agent for the treatment of ophthalmic infections.
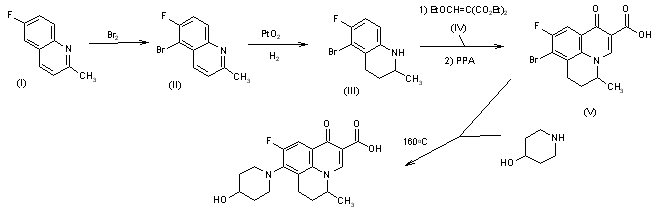
5-Bromo-6-fluoro-2-methylquinoline (II)
5-Bromo-6-fluoro-2-methyl-1,2,3,4-tetrahydroquinoline (III)
diethyl 2-[(E)-ethoxymethylidene]succinate (IV)
8-Bromo-9-fluoro-5-methyl-1-oxo-6,7-dihydro-1H,5H-pyrido[3,2,1-ij]quinoline-2-carboxylic acid (V)
4-Piperidinol; 4-Hydroxypiperidine
______________________________________Elemental Analysis for C.sub.19 H.sub.21 N.sub.2 O.sub.4 F C H N______________________________________Calc'd (%): 63.32 5.87 7.78Found (%): 63.28 5.76 7.89______________________________________
Filed under: GENERIC DRUG, Uncategorized Tagged: GENERIC, Jinofloxacin, NADIFLOXACIN

