DOI: 10.1039/C7GC02913F, Communication
 Open Access
Open Access This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.
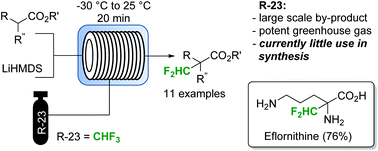
This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.Difluoromethylated esters, malonates and amino acids (including the drug eflornithine) are obtained by a gas-liquid continuous flow protocol employing the abundant waste product fluoroform as an atom-efficient reagent.
Utilization of fluoroform for difluoromethylation in continuous flow: a concise synthesis of α-difluoromethyl-amino acids
Abstract
Fluoroform (CHF3) can be considered as an ideal reagent for difluoromethylation reactions. However, due to the low reactivity of fluoroform, only very few applications have been reported so far. Herein we report a continuous flow difluoromethylation protocol on sp3 carbons employing fluoroform as a reagent. The protocol is applicable for the direct Cα-difluoromethylation of protected α-amino acids, and enables a highly atom efficient synthesis of the active pharmaceutical ingredient eflornithine.
Methyl 3,3-(difluoro)-2,2-diphenylpropanoate (2a) The product mixtures were collected and the solvent removed in vacuo. The products were isolated by thin layer chromatography (dichloromethane/hexane = 3/2 (v/v)). Yield: 173 mg (0.62 mmol, 62%); 93% by 19F NMR ;light yellow viscous liquid. 1 H NMR (300 MHz, D2O): δ = 7.45 – 7.19 (m, 10H), 6.90 (t, 2 JHF = 55.0 Hz, 1H), 3.79 (s, 3H). 13C NMR (75 MHz, D2O): δ = 171.1, 136.3, 129.8, 128.3, 128.2, 115.6 (t, 1 JCF = 246.2 Hz), 64.7, 53.1.19F NMR (282 MHz, D2O):δ = -123.0 (d, 2 JHF = 55.0 Hz).
Conclusions
A gas–liquid continuous flow difluoromethylation protocol employing fluoroform as a reagent was reported. Fluoroform, a by-product of Teflon manufacture with little current synthetic value, is the most attractive reagent for difluoromethylation reactions. The continuous flow process allows this reaction to be performed within reaction times of 20 min with 2 equiv. of base and 3 equiv. of fluoroform. Importantly, the protocol allows the direct Cα-difluoromethylation of protected α-amino acids. These compounds are highly selective and potent inhibitors of pyridoxal phosphate-dependent decarboxylases. The starting materials are conveniently derived from the commercially available α-amino acid methyl esters, and the final products are obtained in excellent purities and yields after simple hydrolysis and precipitation. The developed process appears to be especially appealing for industrial applications, where atom economy, sustainability, reagent cost and reagent availability are important factors.
//////////