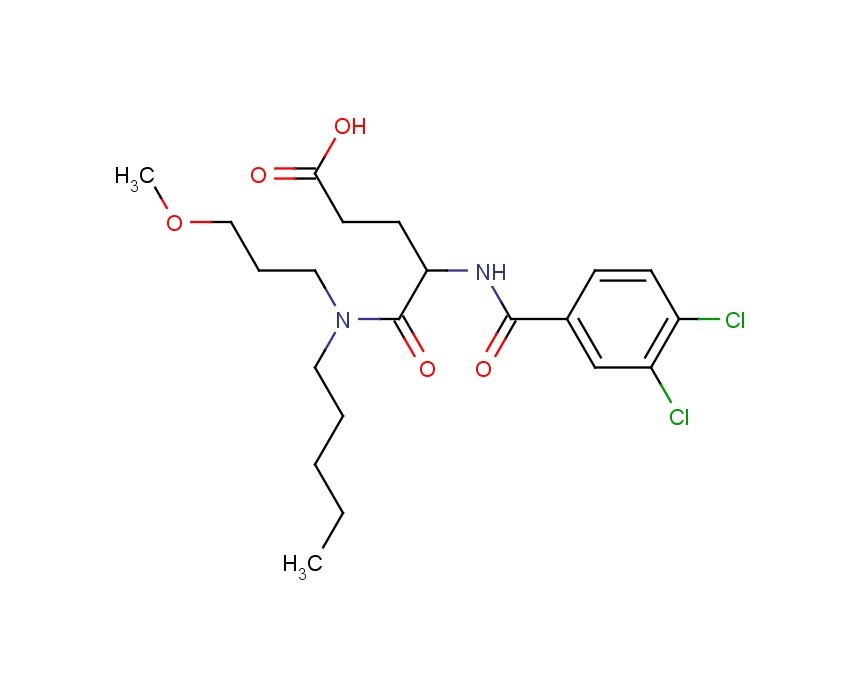
Loxiglumide, CR-1505
molecular formula :C21H30Cl2N2O5
molecular weight 461.3793
CAS NO:107097-80-3
WO 1987003869
Rottapharm (Originator)
Cholecystokinin (CCK) belongs to the group of substances known as brain-gut peptides and function as a neuropeptide and as a gut hormone. (Noble et al., Pharmacol. Rev. 1999, 51(4):745-781; Crawley et al., Peptides 1994, 15(4):731-755). It is now evident that at least two different receptors, namely CCK1 (formerly CCKA or alimentary) and CCK2 (formerly CCKB or brain) receptors, mediate CCK biological actions. (Noble et al., Pharmacol. Rev., 1999, 51(4):745-781; Woodruff and Hughes, Ann. Rev. Pharmacol. 1991, 31:469-501). CCK1 receptors are found in peripheral tissues, including the GI tract.
CCK is secreted primarily in response to meals and plays a well-recognized role in regulating gallbladder contraction and pancreatic enzyme secretion. Over the last decade, considerable evidence has emerged to support the concept that CCK plays an equally important role in the regulation of motor and sensory functions at various levels of the human upper GI tract. Specifically, the native peptide delays gastric emptying, modulates gastric sensory function (especially in response to fat), increases the rate of meal-induced, transient lower esophageal sphincter relaxations (TLESRs) and affects small bowel and colonic transit.
The CCK1 antagonists loxiglumide and dexloxiglumide have demonstrated the ability to reverse the physiologic effects of CCK on gastric emptying and to decrease dyspeptic symptoms induced by air distension and fat infusion. By example,loxiglumide reduced both exogenous and endogenous CCK-induced delay in gastric emptying of liquids and solids in healthy subjects (Borovicka et al., Am J Physiol. 1996, 271:448-453; Schwizer et al., Gut. 1997, 41(4):500-504). Dexloxiglumide reversed the diminished tolerance to water volume that occurred from CCK release in response to duodenal lipid infusion; the effect was due to reduction of intragastric volume, primarily due to accelerated gastric emptying (Lal et al., Am J Physiol Gastrointest Liver Physiol. 2004, 287(1):72-79). When proximal gastric relaxation was produced in healthy subjects by duodenal infusion of lipid, a potent stimulus of CCK release, the relaxation was reversed by loxiglumide (Feinle et al., Gastroenterology 1996, 110(5):1379-1385). Also, loxiglumide modulated antro-pyloroduodenal dysmotility, which is postulated to play a role in generation of dyspeptic symptoms, after it was experimentally induced in healthy subjects by intraduodenal infusion of a mixed liquid meal (Katschinski et al., Eur J Clin Invest. 1996, 26(7):574-583). Loxiglumide was also able to reverse the lowering of intragastric pressure of healthy subjects after duodenal infusion of lipids induced sensations such as fullness and nausea (See Feinle et al., 1996).
In patients with nonulcer dyspepsia and delayed gastric emptying, loxiglumide was shown to accelerate gastric emptying by comparison to placebo (Chua AS, Bekkering M, et al., 1994). Loxiglumide significantly improved dyspeptic symptoms in patients with non-ulcer dyspepsia in an 8-week study (Chua et al., Ann N Y Acad. Sci. 1994, 713:298-299). In another study in patients with functional dyspepsia, aggravation of nausea, fullness, discomfort, bloating and pain was produced by duodenal infusion of lipid with or without balloon distension; dexloxiglumide significantly improved dyspepsia symptom scores compared to placebo (Feinle et al., Gut. 2001, 48(3): 347-355).
Pharmaceutical compositions comprising CCKB antagonists and a proton pump inhibitor to control gastric acid secretion in gastrointestinal disorders have been described in the literature. (See WO 04/098610, WO 04/101533, WO 04/098609, WO 03/041714, WO 01/90078, WO 01/85724, WO 01/85723, WO 01/85704, WO 01/85167, and WO 93/12817) CCK-B receptors mediate CCK biological actions in the brain and are one of several regulators of gastric acid secretion. It is the CCK1 receptors, however, that mediate the CCK biological actions in peripheral tissues including gastric emptying and esophageal sphincter effects.
In addition, combination therapy of a PPI and a second agent, e.g., loxiglumide, to improve impaired esophageal motility has been disclosed as a possible treatment to gastroesophageal reflux disease. (Tonini et al., Drugs 2004, 64(4): 347-361). International Application Nos. PCT/EP2004/050936 and PCT/EP2005/050336 also disclose pharmaceutical combinations of a proton pump inhibitor and a compound that modifies gastrointestinal motility. Both international applications disclose that dexloxiglumide may be useful for therapy of irritable bowel syndrome (IBS) or GERD and may be used to modify gastrointestinal motility.
D,l-4-(3,4-dichlorobenzoylamino)-5-(N-3-methoxypropyl-pentylamino)-5-oxopentanoic acid (CR 1505; loxiglumide) is a newly developed analog of proglumide.

N-(3,4-dichlorobenzoyl)-glutamic acid anhydride (I) is condensed with N-(3-methoxypropyl)-N-pentylamine (II) in water at 5 °C to produce Loxiglumide.


Filed under: Uncategorized Tagged: Loxiglumide
