
SUMATRIPTAN, GR-43175
1-[3-(2-dimethylaminoethyl)-1H-indol-5-yl]- N-methyl-methanesulfonamide
3-[2-(Dimethylamino)ethyl]-N-methyl-1H-indole-5-methanesulfonamide
| Formula | C14H21N3O2S |
|---|---|
| Mol. mass | 295.402 g/mol |
| CAS number | 103628-46-2 |
|---|
Avanir Pharmaceuticals has filed a new drug application (NDA) with the US Food and Drug Administration (FDA) for approval of its new breath-powered investigational drug-device combination product, ‘AVP-825′, for the acute treatment of migraines. click on title Avanir files new drug application for migraine drug
![Sumatriptan molecule]() SUMATRIPTAN
SUMATRIPTAN
SUMATRIPTAN SUCCINATE

AVP-825 is an investigational drug-device combination product consisting of low-dose sumatriptan powder delivered intranasally utilizing a novel Breath Powered delivery technology. If approved, AVP-825 would be the first and only fast-acting, dry-powder intranasal form of sumatriptan for the treatment of migraine.
The Breath Powered delivery technology is activated by user’s breath to propel medications deep into the nasal cavity where absorption is more efficient and consistent than through most other routes. A user exhales into the device, automatically closing the soft palate and sealing off the nasal cavity completely. Through a sealing nosepiece placed into the nostril, the exhaled breath carries medication from the device directly into one side of the nose. Narrow nasal passages are gently expanded and medication is dispersed deep into the nasal cavity reaching areas where it can be rapidly absorbed. As the medication is delivered, the air flows around to the opposite side of the nasal cavity and exits through the other nostril. Closure of the soft palate helps prevent swallowing or inhalation of sumatriptan powder into the lungs.
| Canada | 2469019 | APPROVED 2005-09-13 | EXP 2022-12-04 |
| United States | 6135979 | 1997-03-21 | 2017-03-21 |
| United States | 5705520 | 1994-12-10 | 2011-12-10 |
| Canada | 2098302 | 2001-10-16 | 2011-12-10 |
| Patent No | PatentExpiry | use code |
|---|---|---|
| 5307953 | Dec 2, 2012 | |
| 5307953*PED | Jun 2, 2013 | |
| 5554639 | Sep 10, 2013 | U-232…METHOD OF TREATING MIGRAINE |
| 5554639*PED | Mar 10, 2014 |
Sumatriptan is a synthetic drug belonging to the triptan class, used for the treatment of migraine headaches. Structurally, it is an analog of the naturally occurring neuro-active alkaloids dimethyltryptamine (DMT), bufotenine, and 5-methoxy-dimethyltryptamine, with an N-methyl sulfonamidomethyl- group at position C-5 on the indole ring.[1]
Sumatriptan is produced and marketed by various drug manufacturers with many different trade names such as Sumatriptan, Imitrex, Treximet, Imigran, Imigran recovery.
Large doses of sumatriptan can cause sulfhemoglobinemia, a rare condition in which the blood changes from red to greenish-black, due to the integration of sulfur into the hemoglobin molecule.[2] If sumatriptan is discontinued, the condition reverses within a few weeks.
Serious cardiac events, including some that have been fatal, have occurred following the use of sumatriptan injection or tablets. Events reported have included coronary artery vasospasm, transient myocardial ischemia, myocardial infarction, ventricular tachycardia, and ventricular fibrillation.
The most common side-effects[3] reported by at least 2% of patients in controlled trials of sumatriptan (25, 50, and 100 mg tablets) for migraine are atypical sensations (paresthesias and warm/cold sensations) reported by 4% in the placebo group and 5–6% in the sumatriptan groups, pain and other pressure sensations (including chest pain) reported by 4% in the placebo group and 6–8% in the sumatriptan groups, neurological events (vertigo) reported by less than 1% in the placebo group and less than 1% to 2% in the sumatriptan groups. Malaise/fatigue occurred in less than 1% of the placebo group and 2–3% of the sumatriptan groups. Sleep disturbance occurred in less than 1% in the placebo group to 2% in the sumatriptan group.
![]() SUMATRIPTAN
SUMATRIPTAN
Sumatriptan is structurally similar to serotonin (5HT), and is a 5-HT (types 5-HT1D and 5-HT1B[4]) agonist. The specific receptor subtypes it activates are present on the cranial arteries and veins. Acting as an agonist at these receptors, sumatriptan reduces the vascular inflammation associated with migraines.
The specific receptor subtype it activates is present in the cranial and basilar arteries. Activation of these receptors causes vasoconstriction of those dilated arteries. Sumatriptan is also shown to decrease the activity of the trigeminal nerve, which, it is presumed, accounts for sumatriptan’s efficacy in treating cluster headaches. The injectable form of the drug has been shown to abort a cluster headache within fifteen minutes in 96% of cases.[5]
Sumatriptan is administered in several forms; tablets, subcutaneous injection, and nasal spray. Oral administration (as succinate) suffers from poorbioavailability, partly due to presystemic metabolism—some of it gets broken down in the stomach and bloodstream before it reaches the target arteries. A new rapid-release tablet formulation has the same bioavailability, but the maximum concentration is achieved on average 10–15 minutes earlier. When injected, sumatriptan is faster-acting (usually within 10 minutes), but the effect lasts for a shorter time. Sumatriptan is metabolised primarily by monoamine oxidase A into an indole acetic acid analogue, part of which is further conjugated with glucuronic acid. These metabolites are excreted in the urine and bile. Only about 3% of the active drug may be recovered unchanged.
There is no simple, direct relationship between sumatriptan concentration (pharmacokinetics) per se in the blood and its anti-migraine effect (pharmacodynamics). This paradox has, to some extent, been resolved by comparing the rates of absorption of the various sumatriptan formulations, rather than the absolute amounts of drug that they deliver.[6][7]
Sumatriptan was the first clinically available triptan (in 1991). In the United States, it is available only by medical prescription. However, it can be bought over the counter in the UK and Sweden in 50 mg dosage. Several dosage forms for sumatriptan have been approved, including tablets, solution for injection, and nasal inhalers.
On April 15, 2008, the US FDA approved Treximet, a combination of sumatriptan and naproxen, an NSAID.[8] This combination has shown a benefit over either medicine used separately.[9]
In July 2009, the US FDA approved a single-use jet injector formulation of sumatriptan. The device delivers a subcutaneous injection of 6 mg sumatriptan, without the use of a needle.Autoinjectors with needles have been previously available in Europe and North America for several years.[10]
Phase III studies with a iontophoretic transdermal patch (Zelrix/Zecuity) started in July 2008.[11] This patch uses low voltage controlled by a pre-programmed microchip to deliver a single dose of sumatriptan through the skin within 30 minutes.[12][13]Zecuity was approved by the US FDA in January 2013.[14]
On November 6, 2008, Par Pharmaceutical announced that it would begin shipping generic versions of sumatriptan injection (sumatriptan succinate injection) 4 mg and 6 mg starter kits and 4 mg and 6 mg pre-filled syringe cartridges to the trade immediately. In addition, Par anticipates launching the 6 mg vials early in 2009.[15]
Mylan Laboratories Inc., Ranbaxy, Sandoz, Dr. Reddy’s Pharmaceuticals and other companies have received FDA approval for generic versions of Imitrex tablets in 25-, 50-, and 100-milligram doses since 2009. The drug is available in U.S. and European markets, since Glaxo’s patent protections have expired in those jurisdictions. However, sales of a generic delivered via nasal spray are still restricted in the United States.
See also Sumavel DosePro (above).[10]
Chemistry
hydrogenation of nitrile with pd/c in presence of dimethyl amine
…………………
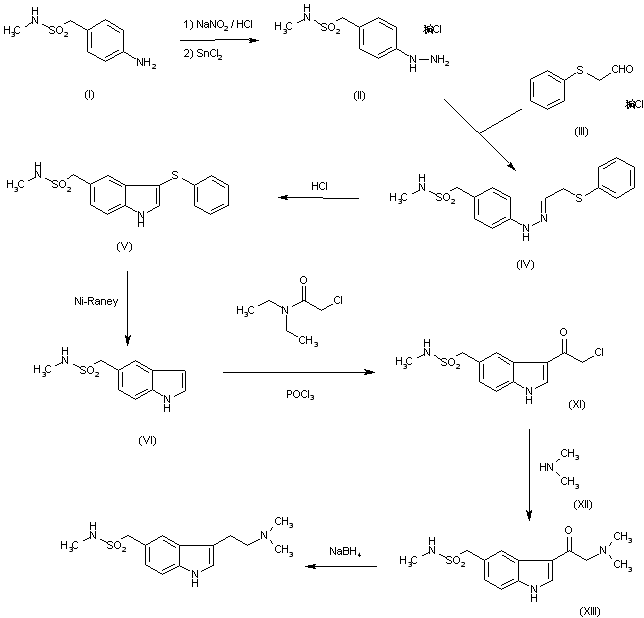
The diazotation of 4-amino-N-methylbenzenemethanesulfonamide (I) with NaNO2-HCl followed by reduction with SnCl2 gives the 4-hydrazino compound (II), which is condensed with (phenylthio)acetaldehyde (III) in ethanol yielding the ethylideneamino compound (IV). The cyclization of (IV) with HCl in ethanol affords N-methyl-3-(phenylthio)-1H-indole-5-methansulfonamide (V), which is desulfurized with RaNi in refluxing ethanol-water to give N-methyl-1H-indole-5-methanesulfonamide (VI). The reaction of (VI) with oxalyl chloride and dimethylamine yields the oxalyl derivative (VII), which is finally reduced with LiAlH4 in refluxing THF.

The condensation of hydrazine (II) with 4,4-dimethoxy-N,N-dimethylbutylamine (VIII) by means of HCl in water gives the butylidenehydrazino compound (IX), which is cyclized with polyphosphate ester (PPE) in CHCl3.

……………………
Beilstein J. Org. Chem. 2011, 7, 442–495.
http://www.beilstein-journals.org/bjoc/single/articleFullText.htm?publicId=1860-5397-7-57#S9
ref are below article
Indoles
The neuroamine transmitter serotonin contains an indole ring, so it is not surprising that indoles are a recurring theme in many drugs affecting central nervous system (CNS) function including antidepressants, antipsychotics, anxiolytics and antimigraine drugs, as well as psychedelic agents. Indole is also one of the best represented heterocyclic motifs present in the top selling pharmaceuticals, being found in eight of the top 200 drugs, with five of these belonging to the triptan family of antimigraine treatments. The classical Fischer indole synthesis is usually reported as one of the first choice routes to prepare these scaffolds. Drugs such as GSK’s serotonin receptor modulators sumatriptan (49, Imitrex) and zolmitriptan (50, Zomig) use the Fischer indole synthesis at a late stage in order to form the desired compound albeit in only low to moderate yields (Scheme 9).
![[1860-5397-7-57-i9]](http://www.beilstein-journals.org/bjoc/content/inline/1860-5397-7-57-i9.png?max-width=550&background=EEEEEE)
However, in sumatriptan the indole product resulting from the Fischer synthesis can still react further which leads to the formation of by-products and significantly reduced yields. One way to minimise this was to protect the nitrogen of the sulfonamide group prior to indole formation [11]. This leads not only to an increased yield in the indole forming step (to 50%) but also facilitates chromatographic purification. The dimethylamino group can be present from the beginning of the synthesis or can be introduced via displacement of chloride or reduction of a cyano moiety. Alternatively, the dimethyl ethylene amine side chain can be introduced in position 3 via a Friedel–Crafts-type acylation. The resulting acid chloride is transformed in situ to the corresponding amide which on reduction with lithium aluminium hydride affords sumatriptan (Scheme 10) [12].
![[1860-5397-7-57-i10]](http://www.beilstein-journals.org/bjoc/content/inline/1860-5397-7-57-i10.png?max-width=550&background=EEEEEE)
In the standard Fischer indole synthesis a hydrazine, which is most commonly derived from the corresponding diazonium salt, is reacted with a suitable carbonyl compound. Alternatively, the Japp–Klingemann reaction can be used to directly couple the diazonium salt with a β-ketoester to obtain a hydrazone which can then undergo indole ring formation (Scheme 11) [13].
![[1860-5397-7-57-i11]](http://www.beilstein-journals.org/bjoc/content/inline/1860-5397-7-57-i11.png?max-width=550&background=EEEEEE)
As can be seen from Scheme 11 the indole 59 prepared via the Japp–Klingemann reaction is substituted at position 2 by an ester group which prevents reaction with electrophiles, thereby reducing the amount of undesired by-products. A simple sequence of hydrolysis and decarboxylation then affords sumatriptan [14].
All the reported methods for the synthesis of sumatriptan begin with the sulfonamide group already present on the aromatic ring and several routes are possible to introduce this functional group. The scalable route to the sulfonamides inevitably involves the preparation of the sulfonyl chloride intermediate which is then trapped with the desired amine. The sulfonyl chloride can also be prepared from the corresponding hemithioacetal 61 by treatment with NCS in wet acetic acid (Scheme 12). This efficient oxidation produces only methanol and formaldehyde as by-products [15].
![[1860-5397-7-57-i12]](http://www.beilstein-journals.org/bjoc/content/inline/1860-5397-7-57-i12.png?max-width=550&background=EEEEEE)
- 11. Pete, B.; Bitter, I.; Szántay, C., Jr.; Schön, I.; Töke, L. Heterocycles 1998, 48, 1139–1149. doi:10.3987/COM-97-8087
- 12…Oxford, A. W. Indole Derivative. U.S. Patent 5,037,845, Aug 6, 1991.
- 13…Japp, F. R.; Klingemann, F. Chem. Ber. 1887, 20, 2942–2944. doi:10.1002/cber.188702002165
- Pete, B.; Bitter, I.; Harsányi, K.; Töke, L. Heterocycles 2000, 53, 665–673. doi:10.3987/COM-99-8815
- Kim, D.-W.; Ko, Y. K.; Kim, S. H. Synthesis 1992, 12, 1203–1204. doi:10.1055/s-1992-26333
[15
References for full article
- The presence of the sulfonamide group in the molecule does not make sumatriptan a "sulfa drug", since it does not have any anti-microbial properties.
- "Patient bleeds dark green blood". BBC News. 8 June 2007. Retrieved 6 March 2010.
- Tablets
- Razzaque Z, Heald MA, Pickard JD, et al. (1999). "Vasoconstriction in human isolated middle meningeal arteries: determining the contribution of 5-HT1B- and 5-HT1F-receptor activation".Br J Clin Pharmacol 47 (1): 75–82. doi:10.1046/j.1365-2125.1999.00851.x. PMC 2014192.PMID 10073743.
- Treatment of acute cluster headache with sumatriptan. The Sumatriptan Cluster Headache Study Group. N Engl J Med 1991;325:322-6.
- Fox, A. W. (2004). "Onset of effect of 5-HT1B/1D agonists: a model with pharmacokinetic validation". Headache 44 (2): 142–147. doi:10.1111/j.1526-4610.2004.04030.x.PMID 14756852. edit
- Freidank-Mueschenborn, E.; Fox, A. (2005). "Resolution of concentration-response differences in onset of effect between subcutaneous and oral sumatriptan". Headache 45 (6): 632–637. doi:10.1111/j.1526-4610.2005.05129a.x. PMID 15953294. edit
- GSK press release – Treximet (sumatriptan and naproxen sodium) tablets approved by FDA for acute treatment of migraine
- Brandes JL, Kudrow D, Stark SR, et al. (April 2007). "Sumatriptan-naproxen for acute treatment of migraine: a randomized trial". JAMA 297 (13): 1443–54.doi:10.1001/jama.297.13.1443. PMID 17405970.
- Brandes, J.; Cady, R.; Freitag, F.; Smith, T.; Chandler, P.; Fox, A.; Linn, L.; Farr, S. (2009). "Needle-free subcutaneous sumatriptan (Sumavel DosePro): bioequivalence and ease of use.". Headache 49 (10): 1435–1444. doi:10.1111/j.1526-4610.2009.01530.x.PMID 19849720. edit
- ClinicalTrials.gov NCT00724815 The Efficacy and Tolerability of NP101 Patch in the Treatment of Acute Migraine (NP101-007)
- SmartRelief -electronically assisted drug delivery (iontophoresis)
- Pierce, M; Marbury, T; O'Neill, C; Siegel, S; Du, W; Sebree, T (2009). "Zelrix: a novel transdermal formulation of sumatriptan". Headache 49 (6): 817–25. doi:10.1111/j.1526-4610.2009.01437.x. PMID 19438727.
- Zecuity Approved by the FDA for the Acute Treatment of Migraine
- "PAR PHARMACEUTICAL BEGINS SHIPMENT OF SUMATRIPTAN INJECTION". Par Pharmaceutical. 2008-11-06. Retrieved 2008-11-25.
- Serotonin 5HT1-receptor agonist. Prepn: M. D. Dowle, I. H. Coates, DE 3320521; eidem, US 4816470; A. W. Oxford, GB 2162522 (1983, 1989, 1986 all to Glaxo).
- Receptor binding studies: P. P. A. Humphrey et al., Br. J. Pharmacol.94, 1123 (1988); P. Schoeffter, D. Hoyer, Arch. Pharmacol. 340, 135 (1989).
- LC-MS determn in plasma: J. Oxford, M. S. Lant, J. Chromatogr. 496, 137 (1989).
- Clinical evaluations in migraine: A. Doenicke et al., Lancet 1, 1309 (1988);
- Subcutaneous Sumatriptan International Study Group, N. Engl. J. Med. 325, 316 (1991); in acute cluster headache: Sumatriptan Cluster Headache Study Group, ibid. 322.
- Review of pharmacology and clinical experience: S. J. Peroutka, Headache 30 (Suppl. 2), 554-560 (1990).
- Drugs Fut 1989,14(1),35
|
1-4-2012
|
Noncardiotoxic pharmaceutical compounds
|
|
|
7-9-2010
|
NON-MUCOADHESIVE FILM DOSAGE FORMS
|
|
|
1-22-2010
|
Fixed Combination Dosage Forms for the Treatment of Migraine
|
|
|
12-11-2009
|
ACTIVE AGENT DELIVERY SYSTEMS AND METHODS FOR PROTECTING AND ADMINISTERING ACTIVE AGENTS
|
|
|
10-9-2009
|
PHARMACEUTICAL COMPOSITIONS COMPRISING A TRIPTAN AND A NONSTEROIDAL ANTI-INFLAMMATORY DRUG
|
|
|
10-9-2009
|
ACTIVE AGENT DELIVERY SYSTEMS AND METHODS FOR PROTECTING AND ADMINISTERING ACTIVE AGENTS
|
|
|
5-7-2009
|
Patient controlled drug delivery device
|
|
|
3-20-2009
|
DEUTERIUM-ENRICHED SUMATRIPTAN
|
|
|
3-13-2009
|
Rapid dissolution of combination products
|
|
|
2-19-2009
|
A METHOD OF IDENTIFYING MODULATORS OF CELL SURFACE MEMBRANE RECEPTORS USEFUL IN THE TREATMENT OF DISEASE
|
|
4-8-1992
|
PREPARATION OF INDOLE DERIVATIVES
|
|
|
1-10-1992
|
PHARMACEUTICAL PREPARATIONS
|
|
|
10-32-1991
|
SYSTEM AND METHOD FOR DETERMINING THREE-DIMENSIONAL STRUCTURES OF PROTEINS
|
|
|
8-7-1991
|
Indole derivative
|
|
|
7-4-1990
|
Pharmaceutical formulations
|
|
|
8-8-1984
|
Fuel and water homogenizer
|
Avanir Pharmaceuticals, Inc. is a biopharmaceutical company focused on bringing innovative medicines to patients with central nervous system disorders of high unmet medical need. As part of our commitment, we have extensively invested in our pipeline and are dedicated to advancing medicines that can substantially improve the lives of patients and their loved ones. For more information about Avanir, please visit http://www.avanir.com.
AVANIR® is a trademark or registered trademark of Avanir Pharmaceuticals, Inc. in the United States and other countries. All other trademarks are the property of their respective owners.
Avanir Pharmaceuticals, Inc. licensed exclusive rights for the development and commercialization of AVP-825, a novel Breath Powered intranasal system containing a low-dose sumatriptan powder from OptiNose Inc. of Yardley, PA.
IMITREX Tablets contain sumatriptan succinate, a selective 5-HT1B/1D receptor agonist. Sumatriptan succinate is chemically designated as 3-[2-(dimethylamino)ethyl]-N-methyl-indole- 5-methanesulfonamide succinate (1:1), and it has the following structure:
IMITREX Tablets contain sumatriptan succinate, a selective 5-HT1B/1Dreceptor agonist. Sumatriptan succinate is chemically designated as 3-[2-(dimethylamino)ethyl]-N-methyl-indole- 5-methanesulfonamide succinate (1:1), and it has the following structure:
 |
The empirical formula is C14H21N3O2S•C4H6O4, representing a molecular weight of 413.5. Sumatriptan succinate is a white to off-white powder that is readily soluble in water and in saline.
Each IMITREX Tablet for oral administration contains 35, 70, or 140 mg of sumatriptan succinate equivalent to 25, 50, or 100 mg of sumatriptan, respectively. Each tablet also contains the inactive ingredients croscarmellose sodium, dibasic calcium phosphate, magnesium stearate, microcrystalline cellulose, and sodium bicarbonate. Each 100-mg tablet also contains hypromellose, iron oxide, titanium dioxide, and triacetin.
Filed under: NDA Tagged: AVANIR, AVP 825, MIGRAINE, NDA, SUMATRIPTAN

 SUMATRIPTAN
SUMATRIPTAN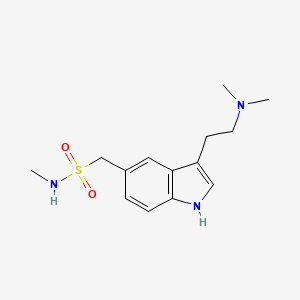 SUMATRIPTAN
SUMATRIPTAN

