
Ezetimibe (1)
(3S,4S)-4-(4-(Benzyloxy)phenyl)-1-(4-fluorophenyl)-3-((S)-3-(4-fluorophenyl)-3′-hydroxypropyl)azetidin-2-one (20)
Method 1


Preparation of 1-(4-Fluorophenyl)-3-(R)-[3-(4-fluorophenyl)-3(S)-hydroxypropyl]-4(S)-(4-hydroxyphenyl)-2-azetidinone 1 (Ezetimibe)
Preparation of (3R,4S)-1-(4-Fluorophenyl)-3-[3-(4-fluorophenyl)-3(S)-hydroxypropyl]-4-(4-benzyloxyphenyl)-2-azetidinone 10
-
Table 2.1H and 13C NMR assignments for Eze-1 and desfluoro Eze-1.
-
Positiona 1H–δ ppm
13C–δ ppm (DEPT)
Eze-1b Desfluoro Eze-1b Eze-1b Desfluoro Eze-1b 1 10.15 (br, OH) 10.13 (br, OH) – – 2 – – 161.3 (C) 161.3 (C) 3 6.87 (d, J=8.5 Hz, 2H) 6.87 (dd, J=8.4, 1.8 Hz, 2H) 116.3 (2CH) 116.3 (2CH) 4 7.74 (d, J=8.5 Hz, 2H) 7.75 (dd, J=8.4, 1.8 Hz, 2H) 131.4 (2CH) 131.4 (2CH) 5 – – 128.1 (C) 128.2 (C) 6 8.43 (s, 1H) 8.43 (s, 1H) 160.8 (CH) 160.8 (CH) 7 – – 149.0 (d, 4J=2.6 Hz, C) 152.7 (C) 8 7.15–7.26 (m, 4H) 7.36 (dd, J=8.1, 7.5 Hz, 2H) 123.3 (d, 3J=8.4 Hz, 2CH) 121.6 (2CH) 9 7.17 (d, J=7.8 Hz, 2H) 116.5 (d, 2J=22 Hz, 2CH) 129.8 (2CH) 10 – 7.18 (t, J=6.3 Hz, 1H) 160.8 (d, 1J=242 Hz, C) 126.0 (CH) - Assignments: s: singlet; d: doublet; t: triplet; m: multiplet; br: broad singlet. Mean values used for coupled signals.
-
- aNumbering of all compounds shown in Fig. 2 and copies of NMR spectra are presented in Appendix A.
- bSolvent is DMSO-d6.
R-Enantiomer in Ezetimibe
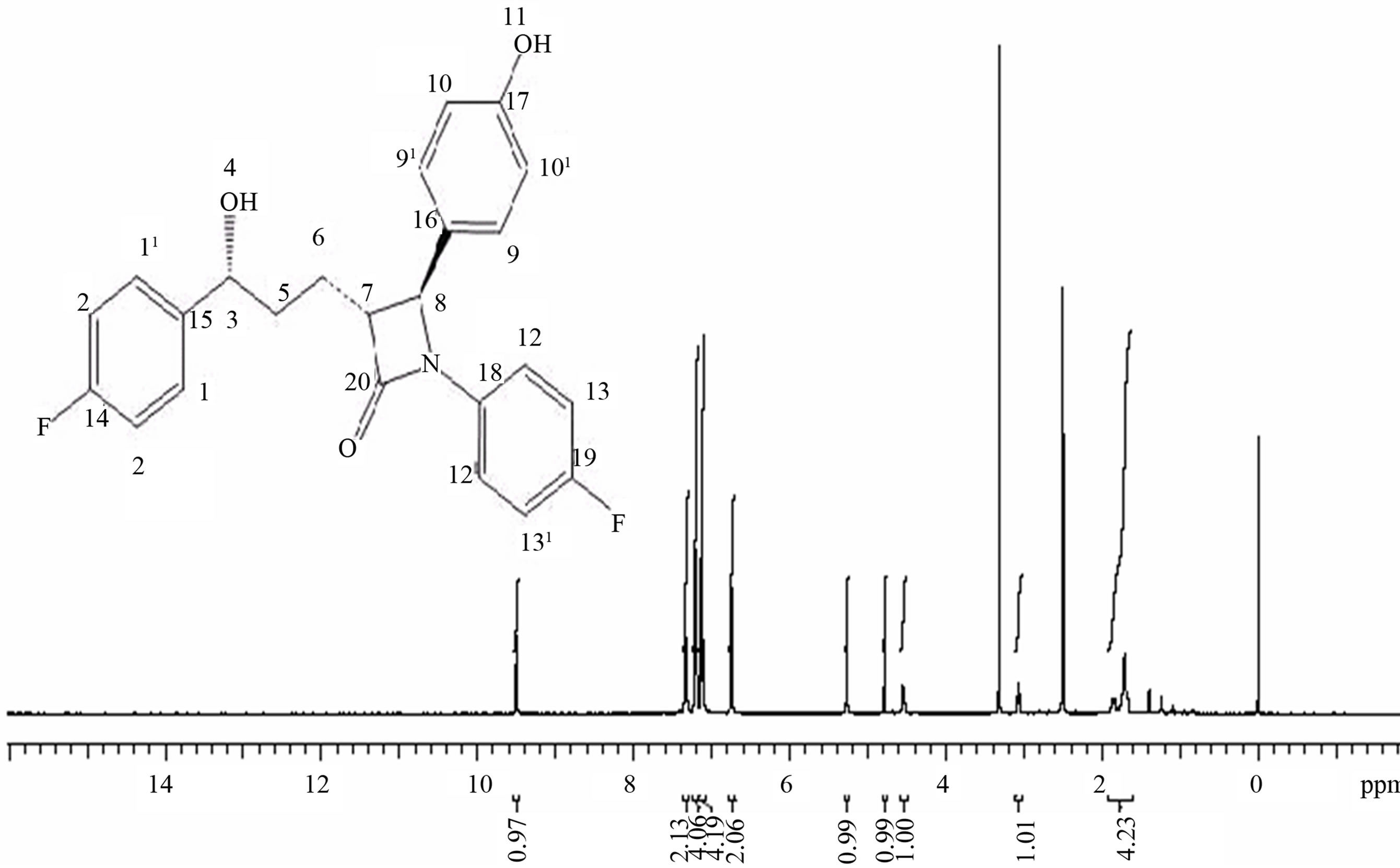
R-Enantiomer in Ezetimibe
ABOVE 1H NMR OF R ENANTIOMER
Isolation and Characterization of R-Enantiomer in Ezetimibe
http://file.scirp.org/Html/10-2200417_36901.htm
1H NMR VALUES FOR R ENANTIOMER

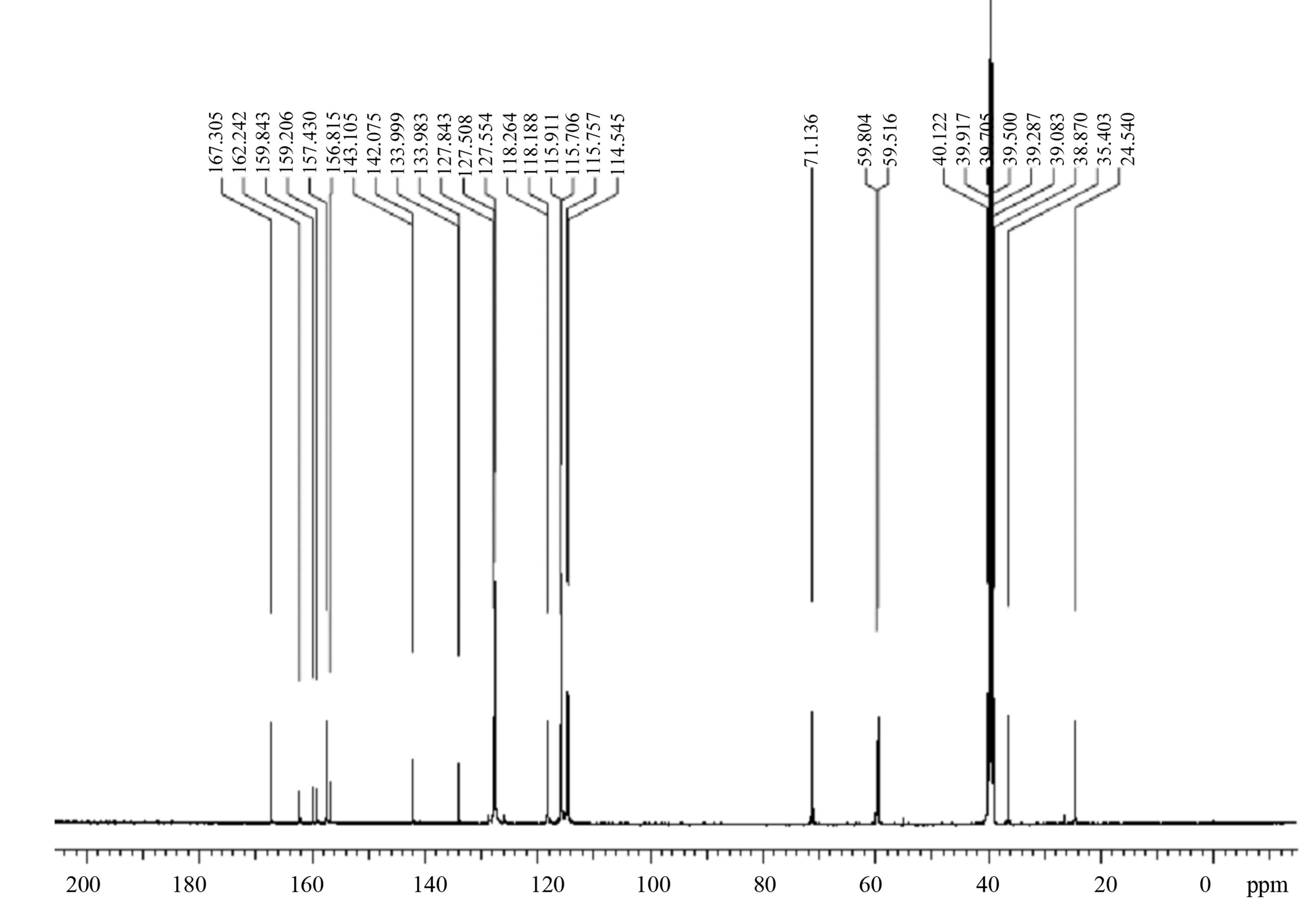
13C NMR VALUES OF R ENANTIOMER


 |
|
| Systematic (IUPAC) name | |
|---|---|
| (3R,4S)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)azetidin-2-one | |
| Clinical data | |
| Trade names | Zetia |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a603015 |
| Legal status | |
| Routes | Oral |
| Pharmacokinetic data | |
| Bioavailability | 35–65% |
| Protein binding | >90% |
| Metabolism | Intestinal wall, hepatic |
| Half-life | 19–30 hours |
| Excretion | Renal 11%, faecal 78% |
| Identifiers | |
| CAS number | 163222-33-1  |
| ATC code | C10AX09 |
| PubChem | CID 150311 |
| DrugBank | DB00973 |
| ChemSpider | 132493  |
| UNII | EOR26LQQ24  |
| KEGG | D01966  |
| ChEBI | CHEBI:49040  |
| ChEMBL | CHEMBL1138  |
| Chemical data | |
| Formula | C24H21F2NO3 |
| Molecular mass | 409.4 g·mol−1 |
| Physical data | |
| Melting point | 164 to 166 °C (327 to 331 °F) |
 (what is this?) (verify) (what is this?) (verify) |
|

1H NMR OF R ENANTIOMER PREDICTED

.

http://www.google.com/patents/US20070049748
EXAMPLE 10 PREPARATION OF 1-(4-FLUOROPHENYL)-3(R)-[3-(4-FLUOROPHENYL)-3(S)-HYDROXYPROPYL]-4(S)-(4-HYDROXYPHENYL)-2-AZETIDINONE (FORMULA I)
EXAMPLE 11 PURIFICATION OF 1-(4-FLUOROPHENYL)-3(R)-[3-(4-FLUOROPHENYL)-3(S)-HYDROXYPROPYL]-4(S)-(4-HYDROXYPHENYL)-2-AZETIDINONE (FORMULA I)
| WO1997045406A1 * | May 28, 1997 | Dec 4, 1997 | Schering Corp | 3-hydroxy gamma-lactone based enantioselective synthesis of azetidinones |
| WO2004099132A2 | May 5, 2004 | Nov 18, 2004 | Ram Chander Aryan | Process for the preparation of trans-isomers of diphenylazetidinone derivatives |
| WO2008032338A2 * | Sep 10, 2007 | Mar 20, 2008 | Reddy Maramreddy Sahadeva | Improved process for the preparation of ezetimibe and its intermediates |
| EP0720599A1 | Sep 14, 1994 | Jul 10, 1996 | Schering Corporation | Hydroxy-substituted azetidinone compounds useful as hypocholesterolemic agents |
| US20070049748 | Aug 25, 2006 | Mar 1, 2007 | Uppala Venkata Bhaskara R | Preparation of ezetim |
| Citing Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| US7470678 | Jul 1, 2003 | Dec 30, 2008 | Astrazeneca Ab | Diphenylazetidinone derivatives for treating disorders of the lipid metabolism |
| US7842684 | Apr 25, 2007 | Nov 30, 2010 | Astrazeneca Ab | Diphenylazetidinone derivatives possessing cholesterol absorption inhibitor activity |
| US7863265 | Jun 19, 2006 | Jan 4, 2011 | Astrazeneca Ab | N-{[4-((2R,3R)-1-(4-fluorophenyl)-3-{[(2R or S)-2-(4-fluorophenyl)-2-hydroxyethyl]thio}-4-oxoazetidin-2-yl)phenoxy]acetyl}glycyl-D-lysine, used as anticholesterol agents |
| US7871998 | Dec 21, 2004 | Jan 18, 2011 | Astrazeneca Ab | Diphenylazetidinone derivatives possessing cholesterol absorption inhibitory activity |
| US7893048 | Jun 21, 2006 | Feb 22, 2011 | Astrazeneca Ab | 2-azetidinone derivatives as cholesterol absorption inhibitors for the treatment of hyperlipidaemic conditions |
| US7906502 | Jun 21, 2006 | Mar 15, 2011 | Astrazeneca Ab | 2-azetidinone derivatives as cholesterol absorption inhibitors for the treatment of hyperlipidaemic conditions |
| US8013150 * | Feb 17, 2006 | Sep 6, 2011 | Msn Laboratories Ltd. | Process for the preparation of ezetimibe |
| US8383810 | Dec 12, 2011 | Feb 26, 2013 | Merck Sharp & Dohme Corp. | Process for the synthesis of azetidinones |
| US20110130378 * | May 26, 2009 | Jun 2, 2011 | Lek Pharmaceuticals D.D. | Ezetimibe process and composition |
| US20110183956 * | Jul 29, 2009 | Jul 28, 2011 | Janez Mravljak | Process for the synthesis of ezetimibe and intermediates useful therefor |
| EP2128133A1 | May 26, 2008 | Dec 2, 2009 | Lek Pharmaceuticals D.D. | Ezetimibe process and composition |
| WO2008096372A2 * | Feb 6, 2008 | Aug 14, 2008 | Pranav Gupta | Process for preparing highly pure ezetimibe using novel intermediates |
| WO2009150038A1 | May 26, 2009 | Dec 17, 2009 | Lek Pharmaceuticals D.D. | Process for the preparation of ezetimibe and composition containing it |
| WO2009157019A2 * | Jun 23, 2009 | Dec 30, 2009 | Ind-Swift Laboratories Limited | Process for preparing ezetimibe using novel allyl intermediates |
| WO2005021497A2 * | Aug 27, 2004 | Mar 10, 2005 | Eduardo J Martinez | Tethered dimers and trimers of 1,4-diphenylazetidn-2-ones |
| WO2006127893A2 * | May 25, 2006 | Nov 30, 2006 | Microbia Inc | Processes for production of 4-(biphenylyl)azetidin-2-one phosphonic acids |
| WO2008096372A2 * | Feb 6, 2008 | Aug 14, 2008 | Pranav Gupta | Process for preparing highly pure ezetimibe using novel intermediates |
| US20070049748 * | Aug 25, 2006 | Mar 1, 2007 | Uppala Venkata Bhaskara R | Preparation of ezetimibe |
Filed under: PROCESS, spectroscopy Tagged: ezetimibe, NMR























