
PART 1…..http://newdrugapprovals.org/2015/04/08/olanzepine/
PART 2….http://newdrugapprovals.org/2015/04/09/olanzepine-visited-part-22/
PART 3…….http://newdrugapprovals.org/2015/04/09/olanzepine-visited-part-33/
review……http://cosmos.ucdavis.edu/archives/2011/cluster8/JAIN_VINEET.pdf
WATCHOUT………..
…………….
DOI: 10.1039/C3OB27424A
http://pubs.rsc.org/en/Content/ArticleLanding/2013/OB/c3ob27424a#!divAbstract
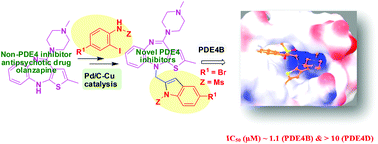
A new strategy for converting antipsychotic drug olanzapine into PDE4 inhibitors is describedvia the design and Pd/C mediated synthesis of novel N-indolylmethyl olanzapine derivatives. One compound showed good inhibition (IC50 1.1 μM) and >10 fold selectivity towards PDE4B over D that was supported by docking studies. This compound also showed significant inhibition of TNF-α and no major toxicities in cell lines and a zebrafish embryo model except the teratogenic effects to be re-assessed in rodents.
…………..
http://www.google.com/patents/US7329747
Olanzapine or 2-methyl-4-[4-methyl-1-piperazinyl]-10H-thieno[2,3b][1,5]-benzodiazepine is a pharmaceutically active compound that can be represented by the formula (1).
It was disclosed in EP 454436 and corresponding U.S. Pat. No. 5,229,382 as a useful antipsychotic agent. Olanzapine acts as a serotonin (5-HT2) and dopamine (D1/D2) receptor antagonist with anticholinergic activity. In commercially available final forms, the active substance is marketed as a free base, which is a white to yellow crystalline solid that is insoluble in water.
One synthetic route for making olanzapine starts from “des-methylpiperazine olanzapine precursor” of formula (3), which reacts with piperazine to form a “des-methyl olanzapine precursor” of formula (2) (see Jun-Da Cen, Chinese Journal of Pharmaceuticals 2001, 32(9),391-393). The compound (2) can be methylated to form olanzapine (see U.S. Pat. No. 4,115,568 for such suggestion). The methylation reaction can be carried out using formaldehyde under conditions of Eschweiler-Clarke reaction (see Jun-Da Cen) or by classical methylation agents such as methyl iodide (see WO 04-000847).
This synthetic pathway has the disadvantage that the reaction with piperazine may lead to formation of dimeric impurities and that the methylation with formaldehyde or other methylation agent may lead to side products, e.g. products of multiple methylation. All these contaminants are difficult to remove from the product. Also, methylation agents are, in general, toxic and mutagenic compounds.
An alternative of the above process was suggested in WO 04/000847 and comprises converting the compound (2) into a “formyl-olanzapine precursor” of formula (4) by a reaction with a methyl formate, and converting the compound (4) into olanzapine by a reduction with a metal borohydride.
In comparison with the preceding procedure, the alternate procedure is one step longer and suffers from the same problems in the step of making compound (2). Furthermore, the reported purity of the actually obtained olanzapine product is only 88%, which is not sufficient for pharmaceutical applications.
The present invention relates to the formation, purification and/or use of an N-formyl olanzapine. Accordingly, a first aspect of the present invention relates to a process, which comprises reacting a des-piperazine olanzapine of formula (3) or a salt thereof
with an N-formyl piperazine of formula (5)
to form an N-formyl olanzapine of formula (4) or a salt thereof
The reaction can be carried out in an inert solvent, generally a dipolar aprotic solvent, and is typically accomplished by heating. The N-formyl olanzapine can be converted to olanzapine.
Another aspect of the invention relates to a process for making an olanzapine salt, which comprises: reducing an N-formyl olanzapine of formula (4) or a salt thereof
with a reducing agent in a solvent to form olanzapine or a salt thereof dissolved in said solvent; reacting said dissolved olanzapine or a salt thereof with an acid to form an acid addition salt of olanzapine; and precipitating said olanzapine acid addition salt from said solution. Precipitating the salt of olanzapine can avoid the formation of technical grade olanzapine. That is, the olanzapine salt can be obtained in a purified state and then converted to olanzapine base, if desired, in high purity.
A further aspect of the present invention relates to purifying the N-formyl olanzapine, which process which comprises:
(1) dissolving and/or slurrying an N-formyl olanzapine of formula (4)
or a salt thereof in a solvent selected from the group consisting of an aliphatic alcohol, an aromatic hydrocarbon, and mixtures thereof, at a temperature of at least 35° C. to form a crystallization treatment medium;
(2) cooling said crystallization treatment medium; and
(3) isolating solid N-formyl olanzapine of formula (4) having improved purity. The steps (1)-(3) can be repeated if necessary until the desired purity is reached. Generally, such a process can achieve purity of greater than 95% and preferably greater than 98%.
An overall synthetic scheme for making olanzapine, which combines various aspects of the present invention, is set forth below:
Example 1
N-Formyl Olanzapine (4)
In a 1000 ml flask, a mixture of 12.0 g of “des-methylpiperazine olanzapine precursor” (compound of formula (2)) hydrochloride and 40 ml of N-formyl piperazine in a mixture of 60 ml of dimethylsulfoxide and 60 ml of toluene was heated at reflux under a nitrogen atmosphere overnight. Progress was monitored by HPLC. After cooling to 40° C., 160 ml of water was added. The resulting mixture was cooled and stirred at 0° C. The solid material was isolated by filtration and washed with 2×40 ml of water. Wet solid was dried overnight at ambient conditions and subsequently at 40° C. under vacuum.
Isolated yield: 12.19 gram, Purity (HPLC): 91.6%
Example 2
Crystallization of the Compound (4)
8.0 g of crude N-formyl olanzapine precursor (compound (4)) of a purity of about 89% (HPLC) was suspended in 50 ml of methanol and heated at 60° C. for 3 hours. The hot suspension was allowed to cool to room temperature and was subsequently cooled to 5° C. under stirring. The solid material was isolated by filtration, washed with 5 ml of cold methanol and 10 ml of cold diethyl ether and dried overnight at 40° C. under vacuum.
Yield: 3.97 g, purity 96.7% (HPLC)
……………….
PATENT
http://www.google.com/patents/WO2004056833A1?cl=en
…………………………….
http://www.google.com/patents/WO2002018390A1?cl=en
Olanzapine is represented by the following structure.
Olanzapine
Olanzapine is useful for treating psychotic patients and mild anxiety states. Preparation of Olanzapine and its acid salts, having pharmaceutical properties particularly in the treatment of disorders ofthe central nervous system has been discussed in U.S. Patent No. 5,229,382.
U.S. Patent No. 5,229,382 does not refer to any specific polymorphic crystalline form of Olanzapine. European patent specification No. 733635A1 claims Form-2 of Olanzapine. The process under this patent describes preparation of Form-2 from ethyl acetate. This patent also designated the product obtained according to the process described in U.S. Patent No. 5,229,382 as Form-1. Furthermore, EP 733635 Al discloses the d values for Form-1 and Form-2 from their X-ray Diffractograms. The values are: d value d value
Form-1 Form-2 9.94 10.26
8.55 8.57 8.24 7.47 6.88 7.12 6.37 6.14 6.24 6.07 5.58 5.48 5.30 5.21 4.98 5.12 4.83 4.98 4.72 4.76 4.62 4.71 4.53 4.47 4.46 4.33 4.29 4.22 4.23 4.14 4.08 3.98 3.82 3.72 3.74 3.56 3.69 3.53 3.58 3.38 3.50 3.25 3.33 3.12 3.28 3.08
3.21 3.06
3.11 3.01
3.05 2.87 2.94 2.81
2.81 ‘ 2.72
2.75 2.64
2.65 2.60
2.63 2.59
It is noteworthy to mention that EP 0 831 098 A2 discloses the preparation of a series of dihydrates of olanzapine namely Dihydrate B, Dihydrate D and Dihydrate E.
The d values from the X-ray Diffractograms for these forms are listed in EP 0 831
098 A2. We conducted experiments to obtain Olanzapine Form I by recrystallization of olanzapine from acetonitrile using the process described in Example 1, sub example 4 of U.S. Patent No. 5,229,382. The process is described herein for reference: A mixture of 4-amino-2-methyl-10H-thieno-[ 2,3-b] [l,5]benzodiazepine HCl (100 g),
N-methyl piperizine (350ml), DMSO (465 ml) and toluene (465 ml) was heated to reflux. The reaction mass was maintained at reflux for 19 hours and then cooled to
50°C and water was added. The reaction mass was cooled to 0-10°C and stirred at the same temperature for 6 hours. The crude Olanzapine separated was filtered and dried in oven to a constant weight (76.5 g). The crude compound was added to acetonitrile (750 ml) at boiling temperature. The mixture was boiled for further 5 minutes. The mixture was filtered to remove the undissolved solid. The filtrate was treated with carbon and filtered. The filtrate was distilled to a minimum volume, cooled to 0-5 °C and maintained at the same temperature for 1.0 hour and filtered.
The compound was dried to a constant weight in an oven (51.6g).
The polymorphic form obtained from these experiments was characterized for its X-ray Powder Diffraction on Rigaku D / Max 2200. As clearly observed, the d values for this product (Fig. 1) matched with those of Olanzapine Form-2 claimed in EP 733635A1. It is therefore inferred that the recrystallization of Olanzapine in acetonitrile produces Form-2 and not Form-1.
Accordingly, the present invention provides a novel method for preparation of hydrates of olanzapine, which are different from those reported in the literature. These hydrates are named Olanzapine monohydrate-I and Olanzapine dihydrate-I for convenience.
Accordingly, the present invention also provides a novel method for preparation of Olanzapine Form-1 by recrystallization of olanzapine or its hydrates in dichloromethane . The present invention also provides a novel method for converting Olanzapine
Form-2 to Olanzapine Form-1
PREPARATION OF OLANZAPINE MONOHYDRATE-1
EXAMPLE 1 A mixture of 4-amino-2-methyl-10H-thieno-[2,3-b][l,5]benzodiazepine hydrochloride (20 Kg), N-methyl piperazine (42 lit), dimethyl sulfoxide (40 lit) and toluene (95 lit) was heated to reflux. The reaction mass was maintained at reflux for
17 hours and 15 minutes and then cooled to 40-50°C. Water (95 lit) was added slowly at40-50°C. The reaction mass was cooled to -0.6 to 1.2°C and stirred at the same temperature for six hours. The Olanzapine crude that separated was filtered and washed with water (10 lit). The product was dried at 30.5 to 31.8°C for 10 hrs and 50 minutes. Yield: 20 Kg. A 20 gm sample from the above material after prolonged heating for an additional 72 hours gave the product with a moisture content of 5.22%.
PREPARATION OF OLANZAPINE DIHYDRATE-I EXAMPLE 2
A mixture of 4-amino-2-methyl-l OH-thieno- [2,3-b] [ 1 ,5]benzodiazepine hydrochloride (200 g), N-methyl piperazine (420 ml), dimethyl sulfoxide (200 ml) and toluene (940 ml) was heated to reflux. The reaction mass was maintained at reflux for 12 hours and then cooled to 40°C. Water (940 ml) was added slowly at 40-44°C. The reaction mass was cooled to 0-5°C and stirred at the same temperature for five hours. The Olanzapine crude that separated was filtered and washed with water (100 ml). The solid obtained was dried atmospherically (25-35°C) for 24 hours (Yield :
241 g).
PREPARATION OF FORM-1
EXAMPLE 3 Crude 2-methyl-4-(4-methyl- 1 -piperazinyl)- 1 OH-thieno- [2,3 -b] [ 1 ,5] benzodiazepine (35.0 g) was suspended in dichloromethane (160.0 ml). The suspension was heated to reflux to obtain a clear solution. The resultant solution was then treated with carbon (3.5 g) followed by filtration. Upon completion of this step the filtrate was cooled to 0 to 5°C and stirred at the same temperature for one hour. The separated solid was filtered and washed with chilled dichloromethane (10.0ml). The product obtained on drying in oven at 65 to 70°C to a constant weight gave Form-1 of Olanzapine (Yield 22.0 g).
CONVERSION OF FORM-2 TO FORM-1 EXAMPLE 4 The stirred suspension of pure form-2 of 2-methyl-4-(4-methyl-l-piperazinyl)-
10H-thieno-[2,3-b][l,5]benzodiazepine (20.0 g) in dichloromethane (90.0 ml) was heated to reflux to obtain a clear solution. The clear solution was filtered and the filtrate was then cooled to 3 to 5°C and stirred at same temperature for one hour. The crystalline solid separated was filtered and washed with dichloromethane (4.0 ml). Subsequent drying at 60 to 70°C to a constant weight yielded Olanzapine Form-1. (Yield: 12.7 g).
PREPARATION OF FORM-1 FROM MONOHYDRATE-I OF OLANZAPINE
EXAMPLE 5 Monohydrate-I of 2-methyl-4-(4-methyl-l-piperazinyl)-10H-thieno-[2,3- b][l,5] benzo- diazepine (25.0 g) prepared as per Example- 1 was suspended in dichloromethane (325.0 ml). The suspension was heated to reflux to obtain a clear solution. The resultant solution was then treated with carbon (2.5 g) followed by filtration. Upon completion of this step the filtrate was distilled to a minimum volume and then cooled to 2 to 4°C and stirred at the same temperature for 90 minutes. The product separated was filtered arid washed with chilled dichloromethane (10 ml). The product obtained on drying in oven at 60 to 70°C to a constant weight gave Form-1 of Olanzapine (Yield 16.5 g)
PREPARATION OF FORM-1 FROM DIHYDRATE-I OF OLANZAPINE
EXAMPLE 6 Dihydrate-I of 2-methyl-4-(4-methyl-l-piperazinyl)-10H-thieno-[2,3-b][l,5] benzodiazepine (40.0 g) prepared as per Example-2 was suspended in dichloromethane (520.0 ml). The suspension was heated to reflux to obtain a clear solution. The resultant solution was then treated with carbon (4.0 g) followed by filtration. Upon completion of this step the filtrate was distilled to a minimum volume and the left over reaction mass was cooled to 0 to 2°C and stirred at the same temperature for one hour. The separated solid was filtered and washed with dichloromethane (10.0ml). The product obtained on drying in oven at 65 to 70°C to a constant weight renders Form-1 of Olanzapine (Yield 26.0 g).
The aforementioned crystalline forms in examples 1 to 6 have been examined for their structural and analytical data viz., Powder X-Ray Diffraction, Differential Scanning Calorimetry, and Infrared Absorption Spectroscopy. The results obtained are discussed and the respective drawings attached (Fig. 2 -19).
………………………………..
http://www.google.com/patents/EP2292624A1?cl=en
-
Olanzapine is a pharmaceutical active substance from the group of antipsychotics, applicable for the treatment of different mental diseases and conditions, such as, for example, disorders of the central nervous system, schizophrenia, hallucination, acute mania, depression, and the like.
-
Olanzapine and analogues thereof are encompassed for the first time within a general formula in the patent GB 1,533,235 and specifically described in EP 454 436 B1 . The patents disclose two different one-step processes for olanzapine preparation. The first described process is a reaction of 4-amino-2-methyl-10H-thieno[2,3-b][1,5]benzodiazepine hydrochloride with N-methylpiperazine in an organic solvent, such as anisole, toluene, dimethyl formamide or dimethyl sulfoxide, preferably at a temperature from 100 to 150°C to yield olanzapine (Scheme 1).
-
The same patent also mentions the formation of acid addition salts of olanzapine and their potential use as intermediates in olanzapine purification process and for a pharmaceutical use.
-
As disclosed in EP 454 436 B1 and US equivalent US 5,229,382 , olanzapine obtained according to the first synthesis (Scheme 1) is purified by recrystallization from acetonitrile, whereas olanzapine prepared according to the second route (Scheme 2) is further purified by column chromatography on Florisil® and recrystallization from acetonitrile. This purification procedure lacks on industrial applicability.
-
Some other synthetic approaches for the preparation of olanzapine describe two steps for creating 4-methylpiperazinyl side chain (Scheme 3). Firstly, 4-amino-2-methyl-10H-thieno [2,3-b][1,5] benzodiazepine hydrochloride reacts with piperazine to yield 2-methyl-4-(1-piperazinyl)-10H-thieno[2,3-b][1,5]benzodiazepine (i.e. N-desmethylolanzapine; Bioorganic & Medicinal Che-mistry Letters, Vol. 7, No. 1, pp. 25-30, 1997), then the methyl group is introduced either by reductive N-methylation (using formaldehyde and metal boron hydride -WO 04/000847) or by nucleophylic substitution reaction with methyl iodide (WO 05/090359). The two-step approach reduces the dark colour appearance but it does not solve the problem of purification from similar by-products.
-
It is well known to a skilled person that most chemical reactions are not completely finished, may be reversible or are driven simultaneously with some other parallel reactions. Starting materials or side reaction products are usually found as impurities in the isolated main product which should therefore be further purified. The simplest way of purification includes various recrystallization and precipitation procedures which are usually less effective if the impurities have physico-chemical properties very similar to the main product.
-
In the case where olanzapine is prepared according to the one step processes disclosed in EP 454 436 B1 , the starting material, 4-amino-2-methyl-10H-thieno[2,3-b][1,5]-benzodiazepine, is found as an impurity in the final product olanzapine.
-
In the case of preparation of olanzapine via the two-step process, as disclosed also in the patent application WO 04/000847 , the presence of 4-amino-2-methyl-10H-thieno[2,3-b][1,5]-benzodiazepine hydrochloride is not critical but various other similar compounds could be found as impurities, such as 4-(4-formylpiperazinyl)-2-methyl-10H-thieno[2,3-b][1,5]-benzo diazepine and N-desmethylolanzapine. In the case of preparation of olanzapine by a two-step process with methyl iodide, an overmethylated N,N-dimethylpiperazinium analogue can be formed.
-
A further undesired impurity which accompanies olanzapine is obtained when olanzapine compound is dissolved in methylene chloride. It is so called olanzapine-CM, being (E)-1-(chloromethyl)-1-methyl-4-(2-methyl-10H-benzo[b]thieno[2,3-e][1,4]diazepin-4-yl)piperazin-1-ium chloride). Olanzapine-CM is formed by alkylation of olanzapine with methylene chloride in methylene chloride solution, for example during evaporation of methylene chloride before the crystallization (Scheme 4).
-
According to the Regulatory Toxicology and Pharmacology 44, pp. 198-211, 2006, classification of potential genotoxic impurities, olanzapine-CM is a potential genotoxic substance due to its R-CH2-Cl structural element, which is known to be involved in reactions with DNA.
-
For all impurities that have a thienobenzodiazepine ring system as a part of the molecule skeleton and because it represents a great part of the molecule, said ring system is crucial for the similarity of physico-chemical properties of said impurities compared to olanzapine.
-
Different salts and crystal forms of an active pharmaceutical ingredient are an important tool for modulating pharmacokinetic properties but can be also a tool for purification.
-
WO 04/089313 discloses olanzapine acid salts, solvates and co-crystals and their use as active pharmaceutical ingredients in formulations. The preparation of fumaric, maleic and malonic acid addition salts of olanzapine is disclosed in WO 04/089313 . Olanzapine acid addition salts disclosed in this application exhibit specific aqueous solubility from 50 µg/ml to 100 mg/ml.
-
WO 05/090359 discloses a method for the purification of olanzapine which has on one hand been prepared by the one-step-process according to Scheme 1, and on the other hand by the process according to Scheme 3, by preparing an addition salt of at least one carboxylic salt, purification of said salt and transfer into purified olanzapine.
-
Neutral olanzapine can be isolated in various crystal forms, hydrates and other solvates. However crystal forms I and II are the most often mentioned as an active pharmaceutical ingredient. Both forms were first disclosed in EP 733 635 alleging that form II is thermodynamically more stable than form I which had been prepared already by the basic patent procedures ( EP 454 436 B1 ).
-
Crystal Growth & Design, Vol. 3, No. 6, pp. 897-907, 2003 discloses anhydrates and hydrates of olanzapine.
-
Olanzapine form I is thermodynamically less stable but it can possess specific kinetic properties which can be applied in designing a final dosage form. Many procedures are known how to prepare it but a person skilled in the art can soon find that the use of methylene chloride is unavoidable to develop a repeatable process. Because this solvent is used in the final step of preparation of olanzapine form I, previous purification methods cannot prevent the presence of the impurity olanzapine-CM in the final product.
-
WO 03/101997 A1 discloses a process for the preparation of a pure olanzapine form I by an addition of methyl-piperazine to the hydrochloride salt of the corresponding benzodiazepine derivative. The purification of olanzapine is conducted by recrystallization. Olanzapine-CM is not disclosed as a disturbing side product which can be removed by recrystallization.
-
WO 02/18390 discloses a process for the preparation of hydrates of olanzapine and the conversion thereof into crystalline forms of olanzapine by recrystallization from methylene dichloride. It is not disclosed that essentially pure olanzapine can be obtained by the removal of olanzapine-CM.
-
WO 04/056833 A1 discloses a process for the preparation of essentially pure olanzapine by removing olanzapine-CM, which is obtained from a solution of olanzapine in methylene chloride, by treating this solution with SiO2, followed by the removal of SiO2. According to the examples, olanzapine is obtained having a purity of 99.92 %, whereas olanzapine-CM is present in an amount of 0.05 %, corresponding to 500 ppm. But SiO2 is acidic, so it adsorbs well also the basic olanzapine what leads to considerable losses of the mother compound.
-
Olanzapin-CM has to be removed in order to obtain essentially pure olanzapine with a high purity which can be further used in pharmaceutical applications. It has been found that olanzapine cannot be efficiently separated from its highly related impurities, in particular from olanzapine-CM, using repeated crystallizations of crude olanzapine. It would therefore be desirable to develop a purification process, in order to provide pharmaceutically acceptable pure and discoloured olanzapine, in particular to provide pharmaceutically acceptable pure olanzapine, being essentially free of the olanzapine-CM impurity. A further object of the present invention is to provide a process for the purification of olanzapine which can also and preferably be conducted in a large scale synthesis.
Example 1 – Synthesis of olanzapine oxalic salt
-
[0093]A solution of 12.0 g of N-desmethylolanzapine in 90 mL of THF and 36 mL of dimethylacetamide (DMAc) is cooled to approx. -20 °C. At -20 °C, 8.19 g of diisopropylamine is added to the solution and afterwards, 8.14 g of methyl iodide is added over 30 min. After stirring the reaction mixture for 65 minutes at -20 °C, 6.4 mL of concentrated hydrochloric acid in 50 mL of water and a solution of 6.36 g of thiourea in 50 mL of water is added and the reaction mixture is stirred for 15 minutes at 20 °C. Then THF is evaporated off and 120 mL of methylene chloride is added and the pH is adjusted to 8.6 with a 40 % water solution of NaOH. After the separation of the phases, the water phase is washed twice with 60 and 30 mL of methylene chloride. The organic phases are combined and 380 mg of acetic anhydride is added and the mixture is stirred for 5 minutes. Then 6.20 g of oxalic acid in 24 mL of methanol is added within 15 minutes. The resulting suspension is stirred for about 1 hour at approx. 20 °C and afterwards 1 hour at approx. 0 °C. The product is isolated by filtration, washed with 100 mL of methylene chloride and dried for 15 hours at 25 °C in vacuo. Yield: 15 g (93 %).
Example 2 – Formation of olanzapine form I
-
[0094]5 g of olanzapine oxalate is dissolved in 50 mL of water and the pH of the solution is adjusted to 2.0 by the addition of 6 N HCl. To the resulting clear solution of olanzapine oxalate, 0.5 g of charcoal is added. After stirring for 5 minutes, the charcoal is filtered off and the cake is washed with 10 mL of water. The filtrate and wash water are combined and after the addition of 60 mL of methylene chloride, the pH of the combined mixture is adjusted to 9.0 by the addition of a 40 % water solution of NaOH. After stirring for 5 minutes, the layers are separated and the water phase is extracted twice with 10 mL of methylene chloride. The organic layers are combined and washed twice with 20 mL of water. After the solution is concentrated in vacuo to the volume of 15 mL, the solution is immediately cooled on an ice/salt bath. The resulting suspension is stirred for 15 minutes, and then olanzapine is isolated by filtration. The wet cake is washed with 3 mL of methylene chloride of the temperature of -20 °C. The product is dried for four hours at 100 °C in vacuo.
HPLC-Purity: 99.9 % olanzapine-CM 380 ppm IR Form I XRD Form I
Example 3 – Formation of olanzapine form I (scale up)
-
[0095]24 kg of olanzapine oxalate is dissolved in 240 L of water and the pH of the solution is adjusted to 2.0 by the addition of 6 N HCl. To the resulting clear solution of olanzapine oxalate, 2.4 kg of charcoal is added. After stirring for 5 minutes, the charcoal is filtered off and the cake is washed with 10 L of water. The filtrate and wash water are combined and after the addition of 300 L of methylene chloride, the pH of the combined mixture is adjusted to 9.0 by the addition of a 40 % water solution of NaOH. After stirring for 15 minutes, the layers are separated and the water phase is extracted twice with 50 L of methylene chloride. The organic layers are combined and washed twice with 100 L of water. After the solution is concentrated in vacuo to the volume of 50 L, the solution is immediately cooled to -15 °C. The resulting suspension is stirred for 30 minutes, then olanzapine is isolated by filtration. The wet cake is washed with 10 L of methylene chloride of the temperature of -20 °C. The product is dried for 10 hours at 100 °C in vacuo.
HPLC-Purity: 99.7 % olanzapine-CM 1000 ppm IR Form I XRD Form I
Example 4 – Formation of olanzapine form I
- (
Al2O3 fluidized bed adsorption
-
[0096]5 g of olanzapine oxalate is dissolved in 50 mL of water and the pH of the solution is adjusted to 2.0 by the addition of 6 N HCl. To the resulting clear solution of olanzapine oxalate, 0.5 g of charcoal is added. After stirring for 5 minutes, the charcoal is filtered off and the cake is washed with 10 mL of water. The filtrate and wash water are combined and after the addition of 60 mL of methylene chloride, the pH of the combined mixture is adjusted to 9.0 by the addition of a 40 % water solution of NaOH. After stirring for 5 minutes, the layers are separated and the water phase is extracted twice with 10 mL of methylene chloride. The organic layers are combined and washed twice with 20 mL of water. After 0.2 g of Al2O3 is added and the methylene chloride suspension is stirred for 5 minutes, Al2O3 is filtered off and the methylene chloride solution is concentrated to the volume of 15 mL. The solution is immediately cooled on an ice/salt bath. The resulting suspension is stirred for 15 minutes, and then olanzapine is isolated by filtration. The wet cake is washed with 3 mL of methylene chloride of the temperature of -20 °C. The product is dried for four hours at 100 °C in vacuo.
HPLC-Purity: 99.9 % olanzapine-CM 214 ppm IR Form I XRD Form I
- )
Example 5 – Formation of olanzapine form I (Al2O3 fluidized bed adsorption
-
[0097]5 g of olanzapine oxalate is dissolved in 50 mL of water and the pH of the solution is adjusted to 2.0 by the addition of 6 N HCl. To the resulting clear solution of olanzapine oxalate, 0.5 g of charcoal is added. After stirring for 5 minutes, the charcoal is filtered off and the cake is washed with 10 mL of water. The filtrate and wash water are combined and after the addition of 60 mL of methylene chloride, the pH of the combined mixture is adjusted to 9.0 by the addition of a 40 % water solution of NaOH. After stirring for 5 minutes, the layers are separated and the water phase is extracted twice with 10 mL of methylene chloride. The organic layers are combined and washed twice with 20 mL of water. Afterwards, the methylene chloride solution is concentrated to 30 mL and 0.2 g of Al2O3is added. After stirring for 5 minutes, Al2O3 is filtered off and the methylene chloride solution is concentrated to the volume of 15 mL. The solution is immediately cooled on an ice/salt bath. The resulting suspension is stirred for 15 minutes, and then olanzapine is isolated by filtration. The wet cake is washed with 3 mL of methylene chloride of the temperature of -20 °C. The product is dried for four hours at 100 °C in vacuo.
HPLC-Purity: 99.9 % Olanzapine-CM 321 ppm IR Form I XRD Form I
- )
Example 6 – Formation of olanzapine form I (Al2O3 fluidized bed adsorption
-
[0098]5 g of olanzapine oxalate is dissolved in 50 mL of water and the pH of the solution is adjusted to 2.0 by the addition of 6 N HCl. To the resulting clear solution of olanzapine oxalate, 0.5 g of charcoal is added. After stirring for 5 minutes, the charcoal is filtered off and the cake is washed with 10 mL of water. The filtrate and wash water are combined and after the addition of 60 mL of methylene chloride, the pH of the combined mixture is adjusted to 9.0 by the addition of a 40 % water solution of NaOH. After stirring for 5 minutes, the layers are separated and the water phase is extracted twice with 10 mL of methylene chloride. The organic layers are combined and washed twice with 20 mL of water. Then the methylene chloride solution is concentrated to 30 mL and 1 g of Al2O3 is added. After stirring for 5 minutes, Al2O3 is filtered off and the methylene chloride solution is concentrated to the volume of 15 mL. The solution is immediately cooled on an ice/salt bath. The resulting suspension is stirred for 15 minutes, and then olanzapine is isolated by filtration. The wet cake is washed with 3 mL of methylene chloride of a temperature of -20 °C. The product is dried for four hours at 100 °C in vacuo.
HPLC-Purity: 99.9 % olanzapine-CM 138 ppm IR Form I XRD Form I
- )
Example 7 – Formation of olanzapine form I (Al2O3 short column adsorption)
-
[0099]5 g of olanzapine oxalate is dissolved in 50 mL of water and the pH of the solution is adjusted to 2.0 by the addition of 6 N HCl. To the resulting clear solution of olanzapine oxalate, 0.5 g of charcoal is added. After stirring for 5 minutes, the charcoal is filtered off and the cake is washed with 10 mL of water. The filtrate and wash water are combined and after the addition of 60 mL of methylene chloride, the pH of the combined mixture is adjusted to 9.0 by the addition of a 40 % water solution of NaOH. After stirring for 5 minutes, the layers are separated and the water phase is extracted twice with 10 mL of methylene chloride. The organic layers are combined and washed twice with 20 mL of water. Then the methylene chloride solution is concentrated to 30 mL and filtered through 20 g of Al2O3 (h = 2 cm). After the methylene chloride solution is concentrated to the volume of 15 mL, the solution is immediately cooled on an ice/salt bath. The resulting suspension is stirred for 15 minutes, and then olanzapine is isolated by filtration. The wet cake is washed with 3 mL of methylene chloride of the temperature of-20 °C. The product is dried for four hours at 100 °C in vacuo.
HPLC-Purity: 99.9 % olanzapine-CM 162 ppm IR Form I XRD Form I
Example 8 – Formation of olanzapine form I (Al2O3 short column adsorption)
-
[0100]5 g of olanzapine oxalate is dissolved in 50 mL of water and the pH of the solution is adjusted to 2.0 by the addition of 6 N HCl. To the resulting clear solution of olanzapine oxalate, 0.5 g of charcoal is added. After stirring for 5 minutes, the charcoal is filtered off and the cake is washed with 10 mL of water. The filtrate and wash water are combined and after the addition of 60 mL of methylene chloride, the pH of the combined mixture is adjusted to 9.0 by the addition of a 40 % water solution of NaOH. After stirring for 5 minutes, the layers are separated and the water phase is extracted twice with 10 mL of methylene chloride. The organic layers are combined and washed twice with 20 mL of water. Afterwards, the methylene chloride solution is concentrated to 30 mL and filtered through 20 g of Al2O3 (h = 5 cm). After the methylene chloride solution is concentrated to the volume of 15 mL, the solution is immediately cooled on an ice/salt bath. The resulting suspension is stirred for 15 minutes, and then olanzapine is isolated by filtration. The wet cake is washed with 3 mL of methylene chloride of the temperature of-20 °C. The product is dried for four hours at 100 °C in vacuo.
HPLC-Purity: 99.9 % olanzapine-CM 73 ppm IR Form I XRD Form I Table 1. Comparison and overview of beneficial effect of Al2O3 treatment
Ex. Scale Treatment Mass (Al2O3) [g] Al2O3 pad height [cm] CM assay [ppm] 2 5 g none / / 380 3 24 kg none / / 1000 4 5 g fluidized bed adsorption 0.2 / 214 5 5 g fluidized bed adsorption (concentrated to 30 mL) 0.2 / 321 6 5 g fluidized bed adsorption (concentrated to 30 mL) 1.0 / 138 7 5 g short column adsorption (concentrated to 30 mL) 20 2 162 8 5 g short column adsorption (concentrated to 30 mL) 20 5 73 Table 2. As comparative data, olanzapine obtained by processes according to the prior art comprises the following amounts of olanzapine-CM:
No. prior art amount of olanzapine-CM 1 WO 2005/090359 , lab. scale 300 – 400 ppm 2 WO 2005/090359 , industrial scale 800 – 2000 ppm 3 WO 2004/056833 <0.15%
………………………………
| Cited Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| EP0454436B1 | Apr 24, 1991 | Sep 13, 1995 | Lilly Industries Limited | Pharmaceutical compounds |
| EP0733635A1 | Mar 22, 1996 | Sep 25, 1996 | Eli Lilly And Company | Crystal forms of a thieno(2,3-B)(1,5) benzodiazepine derivative and process for their preparation |
| GB1533235A | Title not available | |||
| US5229382 | May 22, 1992 | Jul 20, 1993 | Lilly Industries Limited | 2-methyl-thieno-benzodiazepine |
| WO2002018390A1 | Mar 7, 2001 | Mar 7, 2002 | Ramesh Chakka | Process for preparation of hydrates of olanzapine and their conversion into crystalline forms of olanzapine |
| WO2003055438A2 * | Dec 23, 2002 | Jul 10, 2003 | Vijay Chhangamal Chhabada | Crystalline form i of 2-methyl-4-(4-methyl-1-piperazinyl) 10h thieno [2,3-b][1,5]benzodiazepine |
| WO2003101997A1 | May 30, 2003 | Dec 11, 2003 | Geneva Pharmaceuticals Inc | Process of preparation of olanzapine form i |
| WO2004000847A1 | Jun 10, 2003 | Dec 31, 2003 | Adamed Sp Zoo | A process for the preparation of olanzapine and an intermediate therefor |
| WO2004056833A1 | Dec 15, 2003 | Jul 8, 2004 | Adamed Sp Zoo | A process for the preparation of a pharmaceutically pure polymorphic form i of olanzapine |
| WO2004089313A2 | Mar 31, 2004 | Oct 21, 2004 | Magali Bourghol Hickey | Novel olanzapine forms and related methods of treatment |
| WO2005090359A2 | Mar 17, 2005 | Sep 29, 2005 | Lek Pharmaceuticals | Synthesis of 2-methyl-4-(4-methyl-1-piperazinyl)-10h-thieno[2, 3-b][1,5]benzodiazepine and salts thereof |
| WO2008139228A2 * | May 14, 2008 | Nov 20, 2008 | Generics Uk Ltd | Process for the purification of olanzapine |
| Reference | ||
|---|---|---|
| 1 | BIOORGANIC & MEDICINAL CHE-MISTRY LETTERS vol. 7, no. 1, 1997, pages 25 – 30 | |
| 2 | BIOORGANIC & MEDICINAL CHEMISTRY LETTERS vol. 7, no. 1, 1997, pages 25 – 30 | |
| 3 | CRYSTAL GROWTH & DESIGN vol. 3, no. 6, 2003, pages 897 – 907 | |
| 4 | REGULATORY TOXICOLOGY AND PHARMACOLOGY vol. 44, 2006, pages 198 – 211 | |
| WO1998011893A1 * | Sep 18, 1997 | Mar 26, 1998 | Lilly Co Eli | Olanzapine dihydrate d |
| EP0733635A1 * | Mar 22, 1996 | Sep 25, 1996 | Eli Lilly And Company | Crystal forms of a thieno(2,3-B)(1,5) benzodiazepine derivative and process for their preparation |
| EP0831098A2 * | Sep 22, 1997 | Mar 25, 1998 | Eli Lilly And Company | Intermediates and process for preparing olanzapine |
| Citing Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| WO2003037903A1 * | Oct 29, 2002 | May 8, 2003 | Cord Janet I | Olanzapine dihydrate-ii a process for its preparation and use thereof |
| WO2003090730A1 * | Apr 25, 2002 | Nov 6, 2003 | Generics Uk Ltd | Novel crystalline forms of celecoxib and other compounds |
| WO2003091260A1 * | Apr 22, 2003 | Nov 6, 2003 | Ramesh Chakka | Novel crystalline polymorph form-vi of olanzapine and a process for preparation thereof |
| WO2003097650A1 * | May 16, 2003 | Nov 27, 2003 | Urszula Fraczek | Methods for preparation of olanzapine polymorphic form i |
| WO2003101997A1 | May 30, 2003 | Dec 11, 2003 | Geneva Pharmaceuticals Inc | Process of preparation of olanzapine form i |
| WO2004006933A2 | Jul 14, 2003 | Jan 22, 2004 | Krka D D Novo Mesto | Crystal forms of olanzapine and processes for their preparation |
| WO2004056833A1 * | Dec 15, 2003 | Jul 8, 2004 | Adamed Sp Zoo | A process for the preparation of a pharmaceutically pure polymorphic form i of olanzapine |
| WO2004058773A1 * | Dec 24, 2003 | Jul 15, 2004 | Judith Aronhime | Novel crystal forms of olanzapine, methods for their preparation and method for the preparation of known olanzapine crystal forms |
| WO2005070937A1 * | Jan 26, 2005 | Aug 4, 2005 | Rolf Keltjens | A process for making olanzapine in a polymorph form i |
| WO2005080401A1 * | Jul 16, 2004 | Sep 1, 2005 | Shriprakash Dhar Dwivedi | Process for the preparation of olanzapine form 1 useful as antipsychotic drug |
| WO2005107375A2 * | Apr 4, 2005 | Nov 17, 2005 | Jyothi Basu Abbineni | Process for the preparation of olanzapine form-i |
| WO2006006185A1 * | Jul 13, 2005 | Jan 19, 2006 | Saravanan Govindaraju | Improved process for making form i of olanzapine. |
| WO2006025065A1 * | Aug 31, 2004 | Mar 9, 2006 | Lee Pharma Private Ltd | A process for the preparation of anhydrous olanzopine hydrochloride of form-1 |
| WO2008091169A2 | Jan 22, 2008 | Jul 31, 2008 | Tomasz Kozluk Nobilus Ent | Process for preparation of substantially pure polymorphic form i of olanzapine |
| WO2008139228A2 * | May 14, 2008 | Nov 20, 2008 | Generics Uk Ltd | Process for the purification of olanzapine |
| WO2011009831A1 | Jul 19, 2010 | Jan 27, 2011 | Lek Pharmaceuticals D.D. | Process for the purification of olanzapine |
| EP1863775A2 * | Mar 20, 2006 | Dec 12, 2007 | Dr. Reddy’s Laboratories Ltd. | Process for preparing crystalline form i of olanzapine |
| EP1997822A1 * | Nov 10, 2006 | Dec 3, 2008 | EGIS Gyógyszergyár Nyilvánosan Müködõ Részvénytársaság | Process for the preparation of 2-methyl-4-(4-methylpiperazin-1-yl)-10h-thieno-[2,3-b] [1,5] benzodiazepine dihydrochloride trihydrate, 2-methyl-4-(4-methyl-piperazin-1-yl) -10h-thieno[2,3-b] [1,5] benzodiazepine dihydrochloride trihydrate, pharmaceutical compositions comprising it and its use |
| EP2264016A2 | Jul 14, 2004 | Dec 22, 2010 | Jubilant Organosys Limited | A process for producing pure form form of 2-Methyl-4-(4-Methyl-1-Piperazinyl)-10h-thieno[2,3-B][1,5] benzodiazepine |
| EP2292624A1 | Jul 20, 2009 | Mar 9, 2011 | LEK Pharmaceuticals d.d. | Process for the purification of olanzapine |
| US7297789 | May 30, 2003 | Nov 20, 2007 | Sandoz, Inc. | From 4-amino-2-methyl-10H-thieno(2,3-b)(1,5) benzodiazpine HCl and 1-methylpiperazine in an aprotic high boiling solvent; purifying in an acid medium; basifying to pH of 7.5-9; and extracting with a low boiling organic solvent |
| US7323459 | Dec 24, 2003 | Jan 29, 2008 | Teva Pharmaceutical Industries Ltd. | Dissolving olanzapine in a solution of acetic acid and water; filtering the solution; stirring the solution; precipitating the crystalline form by adding a base; and isolating the crystals |
| US7329747 | Jan 27, 2005 | Feb 12, 2008 | Synthon Ip Inc. | Synthesis of olanzapine and intermediates thereof |
| US7425627 | Dec 22, 2004 | Sep 16, 2008 | Teva Pharmaceutical Industries Ltd. | Methods of synthesizing olanzapine |
| US7459449 | Jan 27, 2005 | Dec 2, 2008 | Rolf Keltjens | Olanzapine malonate, olanzapine glycolate, and olanzapine benzoate; antipsychotic agents; water soluble; can form tablets by direct compression; may be mixed with dibasic calcium phosphate and/or hydroxypropyl cellulose |
| US7538213 | May 16, 2003 | May 26, 2009 | Institut Farmaceutyczny | Condensation of molar excess of N-methylpiperazine with 4-amine-2-methyl-10H-thieno [2,3-b][1,5]benzodiazepine hydrochloride in dimethylsulfoxide; recovery and purification with methylene chloride |
| US7745429 | Jul 14, 2003 | Jun 29, 2010 | Krka, D.D. Novo Mesto | Crystal forms of olanzapine and processes for their preparation |
| US7759484 | Jul 7, 2005 | Jul 20, 2010 | Inke, S.A. | Mixed solvate of olanzapine, method for preparing it and method for preparing form I of olanzapine therefrom |
| US7829700 | Sep 5, 2005 | Nov 9, 2010 | Shasun Chemicals And Drugs Limited | Process for preparation of a pharmaceutically pure polymorphic form I of Olanzapine |
| US7834176 | Jan 26, 2006 | Nov 16, 2010 | Sandoz Ag | Polymorph E of Olanzapine and preparation of anhydrous non-solvated crystalline polymorphic Form I of 2-methyl-4(4-methyl-1-piperazinyl)-10H-thieno[2,3-b][1,5] benzodiazepine (Olanzapine Form I) from the polymorphic Olanzapine Form E |
| WO2002018390A1 * | Mar 7, 2001 | Mar 7, 2002 | Ramesh Chakka | Process for preparation of hydrates of olanzapine and their conversion into crystalline forms of olanzapine |
| WO2003097650A1 * | May 16, 2003 | Nov 27, 2003 | Urszula Fraczek | Methods for preparation of olanzapine polymorphic form i |
| WO2003101997A1 * | May 30, 2003 | Dec 11, 2003 | Geneva Pharmaceuticals Inc | Process of preparation of olanzapine form i |
| WO2004006933A2 * | Jul 14, 2003 | Jan 22, 2004 | Krka D D Novo Mesto | Crystal forms of olanzapine and processes for their preparation |
| EP0733367A1 * | Mar 22, 1996 | Sep 25, 1996 | Eli Lilly And Company | Oral olanzapine formulation |
| US5229382 * | May 22, 1992 | Jul 20, 1993 | Lilly Industries Limited | 2-methyl-thieno-benzodiazepine |
| US5703232 * | Jan 16, 1996 | Dec 30, 1997 | Eli Lilly And Company | Process and solvate of 2-methyl-thieno-benzodiazepine |
| US5736541 * | Jul 25, 1996 | Apr 7, 1998 | Eli Lilly And Company | Slurrying impure material in anhydrous ethyl acetate, then crystallization yields pure stable drug for treatment of anxiety, psychological disorders and gastrointestinal disorders |
| US6348458 * | Mar 31, 2000 | Feb 19, 2002 | U & I Pharmaceuticals Ltd. | Polymorphic forms of olanzapine |
| WO2006025065A1 * | Aug 31, 2004 | Mar 9, 2006 | Lee Pharma Private Ltd | A process for the preparation of anhydrous olanzopine hydrochloride of form-1 |
| WO2007052167A2 | Oct 27, 2006 | May 10, 2007 | Actavis Group Ptc Ehf | Stable composition for a pharmaceutical formulation containing olanzapine |
| WO2007144901A1 * | Jun 12, 2007 | Dec 21, 2007 | Jubilant Organosys Ltd | Process for stabilization of olanzapine polymorphic form i |
| WO2008091169A2 | Jan 22, 2008 | Jul 31, 2008 | Tomasz Kozluk Nobilus Ent | Process for preparation of substantially pure polymorphic form i of olanzapine |
| WO2011009831A1 | Jul 19, 2010 | Jan 27, 2011 | Lek Pharmaceuticals D.D. | Process for the purification of olanzapine |
| EP2292624A1 | Jul 20, 2009 | Mar 9, 2011 | LEK Pharmaceuticals d.d. | Process for the purification of olanzapine |
| US7323459 | Dec 24, 2003 | Jan 29, 2008 | Teva Pharmaceutical Industries Ltd. | Dissolving olanzapine in a solution of acetic acid and water; filtering the solution; stirring the solution; precipitating the crystalline form by adding a base; and isolating the crystals |
| US7829700 | Sep 5, 2005 | Nov 9, 2010 | Shasun Chemicals And Drugs Limited | Process for preparation of a pharmaceutically pure polymorphic form I of Olanzapine |


 COCK SAYS MOM CAN TEACH YOU NMR
COCK SAYS MOM CAN TEACH YOU NMR
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE![]() amcrasto@gmail.com
amcrasto@gmail.com
| Cited Patent | Filing date | Publication date | Applicant | Title |
|---|---|---|---|---|
| WO1996038151A1 | May 30, 1995 | Dec 5, 1996 | Lilly Co Eli | Method for treating cognitive dysfunction |
| WO1999016312A1 * | Sep 25, 1998 | Apr 8, 1999 | Lilly Co Eli | Method for treating sexual dysfunction |
| WO2001047933A1 | Dec 22, 2000 | Jul 5, 2001 | Cipla Ltd | New polymorphic forms of olanzapine |
| WO2002018390A1 | Mar 7, 2001 | Mar 7, 2002 | Ramesh Chakka | Process for preparation of hydrates of olanzapine and their conversion into crystalline forms of olanzapine |
| WO2003097650A1 | May 16, 2003 | Nov 27, 2003 | Urszula Fraczek | Methods for preparation of olanzapine polymorphic form i |
| WO2003101997A1 * | May 30, 2003 | Dec 11, 2003 | Geneva Pharmaceuticals Inc | Process of preparation of olanzapine form i |
| WO2004056833A1 | Dec 15, 2003 | Jul 8, 2004 | Adamed Sp Zoo | A process for the preparation of a pharmaceutically pure polymorphic form i of olanzapine |
| WO2005107375A2 * | Apr 4, 2005 | Nov 17, 2005 | Jyothi Basu Abbineni | Process for the preparation of olanzapine form-i |
| WO2006006180A1 * | Jul 14, 2004 | Jan 19, 2006 | Akshat Bhatnagar | A PROCESS FOR PRODUCING PURE FORM OF 2-METHYL-4-(4-METHYL-1-PIPERAZINYL)-10H-THIENO[2,3-b][1,5]BENZODIAZEPINE |
| WO2006010620A2 | Jul 28, 2005 | Feb 2, 2006 | Krka Tovarna Zdravil D D Novo | Olanzapine salts and their conversion to olanzapine free base |
| EP0454436A1 | Apr 24, 1991 | Oct 30, 1991 | Lilly Industries Limited | Pharmaceutical compounds |
| EP0454436B1 | Apr 24, 1991 | Sep 13, 1995 | Lilly Industries Limited | Pharmaceutical compounds |
| EP0733635B1 | Mar 22, 1996 | Aug 16, 2001 | Eli Lilly And Company | Crystal forms of a thieno(2,3-B)(1,5) benzodiazepine derivative and process for their preparation |
| US5637584 | Mar 24, 1995 | Jun 10, 1997 | Eli Lilly And Company | Solvate of olanzapine |
Filed under: Uncategorized Tagged: OLANZEPINE, PART 3/3

















 amcrasto@gmail.com
amcrasto@gmail.com