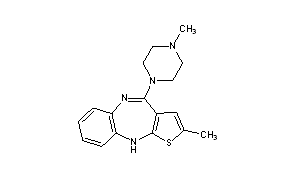

Olanzapine (sold under the brand names Zyprexa, Zypadhera and Lanzek or in combination with fluoxetine, Symbyax) is anatypical antipsychotic. It is approved by the U.S. Food and Drug Administration (FDA) for the treatment of schizophrenia and bipolar disorder.[4]
Olanzapine is structurally similar to clozapine and quetiapine, but is classified as a thienobenzodiazepine. The olanzapine formulations are manufactured and marketed by the pharmaceutical company Eli Lilly and Company; the drug went generic in 2011. Sales of Zyprexa in 2008 were $2.2B in the US, and $4.7B worldwide.[5]
Medical uses
Schizophrenia
The first-line psychiatric treatment for schizophrenia is antipsychotic medication which includes olanzapine.[6] A Cochrane reviewfound that the usefulness for maintenance therapy; however, is difficult to determine as more than half of people in trials quit before the six week completion date.[7]

Zyprexa (olanzapine) 10 mg tablets (AU)
Olanzapine has a higher affinity for 5-HT2A serotonin receptors than D2 dopamine receptors, which is a common property of all atypical antipsychotics, aside from the benzamide antipsychotics such as amisulpride. Olanzapine also had the highest affinity of any second-generation antipsychotic towards the P-glycoprotein in one in vitro study.[60] P-glycoprotein transports a number of drugs across a number of different biological membranes including the blood-brain barrier, which could mean that less brain exposure to olanzapine results from this interaction with the P-glycoprotein.[61]
| Receptor | Ki(nM)[62] | Biologic action and notes[63] |
|---|---|---|
| 5-HT1A | 2282 | Antagonist |
| 5-HT1B | 585 | ? |
| 5-HT1D | 1061 | ? |
| 5-HT1E | 2209 | ? |
| 5-HT2A | 2.4 | Inverse agonist. May underlie the “atypicality” of the newer antipsychotics like olanzapine. May contribute to sedating effects. |
| 5-HT2B | 11.9 | Inverse agonist/antagonist. |
| 5-HT2C | 10.2 | Inverse agonist. May underlie the appetite-stimulating effects of olanzapine. |
| 5-HT3 | 202 | Antagonist. Possibly responsible, at least in part, for its antiemetic action. |
| 5-HT5A | 1212 | ? |
| 5-HT6 | 8.07 | Antagonist |
| 5-HT7 | 105.2 | Antagonist |
| α1A | 112 | Antagonist. Likely responsible for the orthostatic hypotension seen with its use.[63] |
| α1B | 263 | Antagonist. |
| α2A | 315 | Antagonist. |
| α2B | 81.8 | Antagonist |
| α2C | 28.9 | Antagonist. |
| M1 | 26 | Antagonist. Likely the chief receptor responsible for the anticholinergic effects seen with olanzapine’s use.[63] |
| M2 | 63.5 | Antagonist. |
| M3 | 52.67 | Antagonist. Possible role in type 2 diabetes side-effects [64] |
| M4 | 17.33 | Antagonist. |
| M5 | 7.5 | Antagonist. |
| D1 | 70.33 | Antagonist. |
| D2 | 3.00 | Antagonist. Likely responsible for the therapeutic effects of olanzapine against the positive symptoms of schizophrenia.[63] |
| D2Long | 31 | Antagonist. |
| D2Short | 28.77 | Antagonist. |
| D3 | 47 | Antagonist. |
| D4 | 14.33 | Antagonist. |
| D5 | 82 | Antagonist. |
| H1 | 2.19 | Inverse agonist. Likely responsible for the sedative effects of olanzapine.[63] |
| H2 | 44 | Antagonist. |
| H4 | >10000 | Antagonist. |
Olanzapine is a potent antagonist of the muscarinic M3 receptor,[65] which may underlie its diabetogenic side effects.[64][66] Additionally, olanzapine also exhibits a relatively low affinity for serotonin 5-HT1, GABAA, beta-adrenergic receptors, and benzodiazepine binding sites.[67] [27]
The mode of action of olanzapine’s antipsychotic activity is unknown. It may involve antagonism of dopamine and serotonin receptors. Antagonism of dopamine receptors is associated with extrapyramidal effects such as tardive dyskinesia(TD), and with therapeutic effects. Antagonism of muscarinic acetylcholine receptors is associated withanticholinergic side effects such as dry mouth and constipation, in addition it may suppress or reduce the emergence of extrapyramidal effects for the duration of treatment, however it offers no protection against the development of tardive dyskinesia. In common with other second generation (atypical) antipsychotics, olanzapine poses a relatively low risk of extrapyramidal side effects including TD, due to its high affinity for the D1 receptor over the D2 receptor.[68]
Antagonizing H1 histamine receptors causes sedation and may cause weight gain, although antagonistic actions at serotonin 5-HT2C and dopamine D2 receptors have also been associated with weight gain and appetite stimulation.[69]
Dosage forms[edit]
Olanzapine is marketed in a number of countries, with tablets ranging from 2.5 to 20 milligrams. Zyprexa (and generic olanzapine) is available as an orally-disintegrating “wafer” which rapidly dissolves in saliva. It is also available in 10 milligram vials for intramuscular injection.[4]
Metabolism[edit]
Olanzapine is metabolized by the cytochrome P450 system; principally by isozyme 1A2 and to a lesser extent by 2D6. By these mechanisms more than 40% of the oral dose, on average, is removed by the hepatic first-pass effect.[27] Drugs or agents that increase the activity of CYP1A2, notably tobacco smoke, may significantly increase hepatic first-pass clearance of Olanzapine; conversely, drugs which inhibit 1A2 activity (examples: Ciprofloxacin, Fluvoxamine) may reduce Olanzapine clearance.[4]
Society and culture[edit]
Regulatory status[edit]
Olanzapine is approved by the Food and Drug Administration (FDA) for:
- Treatment — in combination with fluoxetine — of depressive episodes associated with bipolar disorder (December 2003).[70]
- Long-term treatment of bipolar I disorder (January 2004).[71][72]
- Treatment — in combination with fluoxetine — of resistant depression (March 2009).[73]
- Oral formulation: acute and maintenance treatment of schizophrenia in adults, acute treatment of manic or mixed episodes associated with bipolar I disorder (monotherapy and in combination with lithium or sodium valproate)
- Intramuscular formulation: acute agitation associated with schizophrenia and bipolar I mania in adults
- Oral formulation combined with fluoxetine: treatment of acute depressive episodes associated with bipolar I disorder in adults, or treatment of acute, resistant depression in adults [74]
- Short-term treatment of acute manic episodes associated with bipolar I disorder (March 2000).[76]
- Short-term treatment of schizophrenia instead of the management of the manifestations of psychotic disorders (March 2000).[76]
- Maintaining treatment response in schizophrenic patients who had been stable for approximately eight weeks and were then followed for a period of up to eight months (November 2000).[76]
Controversy, prosecution, lawsuits and settlements[edit]
Eli Lilly has faced many lawsuits by from people who claimed they developed diabetes or other diseases after taking Zyprexa. In 2006, Lilly paid $700 million to settle 8,000 of these lawsuits.[77] In 2007, Eli Lilly agreed to pay up to $500 million to settle 18,000 more lawsuits.[citation needed]
In 2009 Eli Lilly pled guilty to a criminal misdemeanor charge of illegally marketing Zyprexa for off-label use and agreed to pay $1.4 billion.[78][79]
A New York Times article based on leaked company documents concluded that the company had engaged in a deliberate effort to downplay olanzpine’s side effects.[80] The company denied these allegations and stated that the article had been based on cherry picked documents. Most of the documents were disclosed as the result of lawsuits by individuals who had taken the drug, though other documents had been stolen.[81] Eli Lilly filed a protection order to stop the dissemination of some of the documents which the judge believed to be confidential and “not generally appropriate for public consumption”.[81] Temporary injunctions required those who had received the documents to return them and to remove them from websites.[82] Judge Jack B. Weinstein issued a permanent judgement against further dissemination of the documents and requiring their return by a number of parties named by Lilly.[81] On January 8, 2007, Judge Jack B. Weinstein refused the Electronic Frontier Foundation‘s motion to stay his order.[83] The documents given to The New York Times by Jim Gottstein show that senior Lilly executives may have kept important information from doctors about Zyprexa’s links to obesity and its tendency to raise blood sugar — both known risk factors for diabetes.[citation needed] The Times of London also reported that as early as 1998, Lilly considered the risk of drug-induced obesity to be a “top threat” to Zyprexa sales.[84] On October 9, 2000, senior Lilly research physician Robert Baker noted that an academic advisory board he belonged to was “quite impressed by the magnitude of weight gain on olanzapine and implications for glucose.”[84]
Research[
Olanzapine has been investigated for use as an antiemetic, particularly for the control of chemotherapy-induced nausea and vomiting (CINV). A 2007 study demonstrated its successful potential for this use, achieving a complete response in the acute prevention of nausea and vomiting in 100% of patients treated with moderately and highly-emetogenic chemotherapy, when used in combination with palonosetron and dexamethasone.[85]
Olanzapine has been considered as part of an early psychosis approach for schizophrenia. The Prevention through Risk Identification, Management, and Education (PRIME) study, funded by the National Institute of Mental Health and Eli Lilly, tested the hypothesis that olanzapine might prevent the onset of psychosis in people at very high risk forschizophrenia. The study examined 60 patients with prodromalschizophrenia, who were at an estimated risk of 36–54% of developing schizophrenia within a year, and treated half with olanzapine and half with placebo.[86] In this study, patients receiving olanzapine did not have a significantly lower risk of progressing to psychosis. Olanzapine was effective for treating the prodromal symptoms, but was associated with significant weight gain.[87]
References
- Jump up^ Burton, Michael E.; Shaw, Leslie M.; Schentag, Jerome J.; Evans, William E. (May 1, 2005). Applied Pharmacokinetics & Pharmacodynamics: Principles of Therapeutic Drug Monitoring (4th ed.). Lippincott Williams & Wilkins. p. 815. ISBN 978-0-7817-4431-7.
- ^ Jump up to:a b c d e “PRODUCT INFORMATION OLANZAPINE SANDOZ® 2.5mg/5mg/7.5mg/10mg/15mg/20mg FILM-COATED TABLETS” (PDF). TGA eBusiness Services. Sandoz Pty Ltd. 8 June 2012. Retrieved 26 November 2013.
- ^ Jump up to:a b “Zyprexa, Zyprexa Relprevv (olanzapine) dosing, indications, interactions, adverse effects, and more”. Medscape Reference. WebMD. Retrieved 26 November 2013.
- ^ Jump up to:a b c “Olanzapine Prescribing Information” (PDF). Eli Lilly and Company. 2009-03-19. Retrieved 2009-09-06.
- Jump up^ “Lilly 2008 Annual Report” (PDF). Lilly. 2009. Retrieved 2009-08-06.
- Jump up^ National Collaborating Centre for Mental Health (25 March 2009). “Schizophrenia: Full national clinical guideline on core interventions in primary and secondary care”. Retrieved 25 November 2009.
- Jump up^ Duggan L, Fenton M, Dardennes RM, El-Dosoky A, Indran S (2005). Duggan, Lorna, ed. “Olanzapine for schizophrenia”. Cochrane Database of Systematic Reviews (2): CD001359.doi:10.1002/14651858.CD001359.pub2. PMID 10796640.
- Jump up^ “Psychosis and schizophrenia in adults: treatment and management | Guidance and guidelines | NICE”. National Institute for Health and Care Excellence.
- Jump up^ Barnes TR (2011). “Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology”. J. Psychopharmacol. (Oxford) 25 (5): 567–620. doi:10.1177/0269881110391123.PMID 21292923.
- Jump up^ Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, Thibaut F, Möller HJ (2013). “World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects”. World J. Biol. Psychiatry 14 (1): 2–44. doi:10.3109/15622975.2012.739708. PMID 23216388.
- Jump up^ Abou-Setta, AM; Mousavi, SS; Spooner, C; Schouten, JR; Pasichnyk, D; Armijo-Olivo, S; Beaith, A; Seida, JC; Dursun, S; Newton, AS; Hartling, L (August 2012).PMID 23035275. Missing or empty
|title=(help) - Jump up^ Zhang JP, Gallego JA, Robinson DG, Malhotra AK, Kane JM, Correll CU (July 2013).“Efficacy and safety of individual second-generation vs. first-generation antipsychotics in first-episode psychosis: a systematic review and meta-analysis”. Int. J. Neuropsychopharmacol. 16 (6): 1205–18. doi:10.1017/S1461145712001277.PMC 3594563. PMID 23199972.
- Jump up^ Citrome L (August 2012). “A systematic review of meta-analyses of the efficacy of oral atypical antipsychotics for the treatment of adult patients with schizophrenia”. Expert Opin Pharmacother 13 (11): 1545–73. doi:10.1517/14656566.2011.626769.PMID 21999805.
- Jump up^ Lepping P, Sambhi RS, Whittington R, Lane S, Poole R (May 2011). “Clinical relevance of findings in trials of antipsychotics: systematic review”. Br J Psychiatry 198 (5): 341–5.doi:10.1192/bjp.bp.109.075366. PMID 21525517.
- Jump up^ Désaméricq G, Schurhoff F, Meary A et al. (February 2014). “Long-term neurocognitive effects of antipsychotics in schizophrenia: a network meta-analysis”. Eur. J. Clin. Pharmacol. 70 (2): 127–34. doi:10.1007/s00228-013-1600-y. PMID 24145817.
- Jump up^ Leucht S, Cipriani A, Spineli L et al. (September 2013). “Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis”.Lancet 382 (9896): 951–62. doi:10.1016/S0140-6736(13)60733-3. PMID 23810019.
- Jump up^ Osser DN, Roudsari MJ, Manschreck T (2013). “The psychopharmacology algorithm project at the Harvard South Shore Program: an update on schizophrenia”. Harv Rev Psychiatry 21 (1): 18–40. doi:10.1097/HRP.0b013e31827fd915. PMID 23656760.
- ^ Jump up to:a b c [+http://www.nice.org.uk/guidance/cg185/chapter/1-recommendations “Bipolar disorder: the assessment and management of bipolar disorder in adults, children and young people in primary and secondary care | 1-recommendations | Guidance and guidelines | NICE”].
- Jump up^ Yatham LN, Kennedy SH, O’Donovan C et al. (December 2006). “Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines for the management of patients with bipolar disorder: update 2007″. Bipolar Disord 8 (6): 721–39. doi:10.1111/j.1399-5618.2006.00432.x. PMID 17156158.
- Jump up^ Selle V, Schalkwijk S, Vázquez GH, Baldessarini RJ (March 2014). “Treatments for acute bipolar depression: meta-analyses of placebo-controlled, monotherapy trials of anticonvulsants, lithium and antipsychotics”. Pharmacopsychiatry 47 (2): 43–52.doi:10.1055/s-0033-1363258. PMID 24549862.
- Jump up^ Maglione M, Maher AR, Hu J et al. (2011). “Off-Label Use of Atypical Antipsychotics: An Update”. PMID 22132426.
- Jump up^ Review of olanzapine in the management of bipolar disorders Neuropsychiatr Dis Treat. 2007 October; 3(5): 579–587.
- Jump up^ Scott, Lisa (Winter 2006). “Genetic and Neurological Factors in Stuttering”. Stuttering Foundation of America.
- Jump up^ “Important Safety Information for Olanzapine”. Zyprexa package insert. Eli Lilly & Company. 2007. Archived from the original on 2007-11-23. Retrieved 2007-12-03.
Elderly patients with dementia-related psychosis treated with atypical antipsychotic drugs are at an increased risk of death compared to placebo. […] ZYPREXA (olanzapine) is not approved for the treatment of elderly patients with dementia-related psychosis.
- Jump up^ “Doctors ‘ignoring drugs warning'”. BBC News. 17 June 2008. Retrieved 2008-06-22.
- Jump up^ Leucht S, Cipriani A, Spineli L, Mavridis D, Örey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. The Lancet [Internet]. 2013 Jun 27 [cited 2013 Sep 19];382(9896) 951–62. Available from:http://www.sciencedirect.com/science/article/pii/S0140673613607333
- ^ Jump up to:a b c Lexi-Comp Inc. (2010) Lexi-Comp Drug Information Handbook 19th North American Ed. Hudson, OH: Lexi-Comp Inc. ISBN 978-1-59195-278-7.
- Jump up^ Stöllberger, C., Lutz, W. and Finsterer, J. (2009), Heat-related side-effects of neurological and non-neurological medication may increase heatwave fatalities. European Journal of Neurology, 16: 879–882.
- Jump up^ “OLANZAPINE (olanzapine) tablet OLANZAPINE (olanzapine) tablet, orally disintegrating [Prasco Laboratories]”. DailyMed. Prasco Laboratories. September 2013. Retrieved26 November 2013.
- Jump up^ “Olanzapine 10 mg tablets – Summary of Product Characteristics (SPC)”. electronic Medicines Compendium. Aurobindo Pharma – Milpharm Ltd. 17 May 2013. Retrieved26 November 2013.
- Jump up^ Cerner Multum Incorporated (27 September 2011). “Olanzapine”. Drugs.com.
- Jump up^ Ramankutty G (2002). “Olanzapine-induced destabilization of diabetes in the absence of weight gain”. Acta Psychiatrica Scandinavica 105 (3): 235–6; discussion 236–7.doi:10.1034/j.1600-0447.2002.2c257a.x. PMID 11939979.
- Jump up^ Lambert MT, Copeland LA, Sampson N, Duffy SA (2006). “New-onset type-2 diabetes associated with atypical antipsychotic medications”. Progress in Neuro-Psychopharmacology and Biological Psychiatry 30 (5): 919–23.doi:10.1016/j.pnpbp.2006.02.007. PMID 16581171.
- Jump up^ Moyer, Paula (Oct 25, 2005). “CAFE Study Shows Varying Benefits Among Atypical Antipsychotics”. Medscape Medical News (WebMD). Retrieved 2007-12-03.
- ^ Jump up to:a b AstraZeneca Pharmaceuticals (4 April 2006). “Efficacy and Tolerability of Olanzapine, Quetiapine and Risperidone in the Treatment of First Episode Psychosis: A Randomised Double Blind 52 Week Comparison”. AstraZeneca Clinical Trials. AstraZeneca PLC. Archived from the original on 2007-11-13. Retrieved 2007-12-03.
At week 12, the olanzapine-treated group had more weight gain, a higher increase in [ body mass index ], and a higher proportion of patients with a BMI increase of at least 1 unit compared with the quetiapine and risperidone groups (p<=0.01).
- Jump up^ Wirshing DA, Wirshing WC, Kysar L, Berisford MA, Goldstein D, Pashdag J, Mintz J, Marder SR (1999). “Novel Antipsychotics”. The Journal of Clinical Psychiatry 60 (6): 358–63. doi:10.4088/JCP.v60n0602. PMID 10401912.
- Jump up^ “NIMH study to guide treatment choices for schizophrenia” (Press release). National Institute of Mental Health. 19 September 2005. Retrieved 2006-12-18.
- Jump up^ McEvoy JP, Lieberman JA, Perkins DO, Hamer RM, Gu H, Lazarus A, Sweitzer D, Olexy C, Weiden P, Strakowski SD (2007). “Efficacy and Tolerability of Olanzapine, Quetiapine, and Risperidone in the Treatment of Early Psychosis: A Randomized, Double-Blind 52-Week Comparison”. American Journal of Psychiatry 164 (7): 1050–60.doi:10.1176/appi.ajp.164.7.1050. PMID 17606657.
- Jump up^ Nemeroff CB (1997). “Dosing the antipsychotic medication olanzapine”. The Journal of Clinical Psychiatry 58 (Suppl 10): 45–9. PMID 9265916.
- Jump up^ Fulbright, A. R.; Breedlove, K. T. (2006). “Complete Resolution of Olanzapine-Induced Diabetic Ketoacidosis”. Journal of Pharmacy Practice 19 (4): 255–8.doi:10.1177/0897190006294180.
- Jump up^ Chiu CC, Chen CH, Chen BY, Yu SH, Lu ML (2010). “The time-dependent change of insulin secretion in schizophrenic patients treated with olanzapine”. Progress in Neuro-Psychopharmacology and Biological Psychiatry 34 (6): 866–70.doi:10.1016/j.pnpbp.2010.04.003. PMID 20394794.
- Jump up^ Sacher J, Mossaheb N, Spindelegger C, Klein N, Geiss-Granadia T, Sauermann R, Lackner E, Joukhadar C, Müller M, Kasper S (2007). “Effects of Olanzapine and Ziprasidone on Glucose Tolerance in Healthy Volunteers”. Neuropsychopharmacology 33(7): 1633–41. doi:10.1038/sj.npp.1301541. PMID 17712347.
- Jump up^ Sowell M, Mukhopadhyay N, Cavazzoni P, Carlson C, Mudaliar S, Chinnapongse S, Ray A, Davis T, Breier A, Henry RR, Dananberg J (2003). “Evaluation of Insulin Sensitivity in Healthy Volunteers Treated with Olanzapine, Risperidone, or Placebo: A Prospective, Randomized Study Using the Two-Step Hyperinsulinemic, Euglycemic Clamp”. Journal of Clinical Endocrinology & Metabolism 88 (12): 5875–80. doi:10.1210/jc.2002-021884.PMID 14671184.
- Jump up^ Carey, Benedict (September 20, 2005). “Little Difference Found in Schizophrenia Drugs”. The New York Times. Retrieved 2007-12-03.
- Jump up^ de Haan L, van Amelsvoort T, Rosien K, Linszen D (2004). “Weight loss after switching from conventional olanzapine tablets to orally disintegrating olanzapine tablets”.Psychopharmacology 175 (3): 389–90. doi:10.1007/s00213-004-1951-2.PMID 15322727.
- ^ Jump up to:a b c Taylor, D. The Maudsley prescribing guidelines in psychiatry. Wiley-Blackwell.
- Jump up^ Rasmussen SA, Chu SY, Kim SY, Schmid CH, Lau J (June 2008). “Maternal obesity and risk of neural tube defects: a metaanalysis”. American Journal of Obstetrics & Gynecology198 (6): 611–619. doi:10.1016/j.ajog.2008.04.021. PMID 18538144.
- Jump up^ McMahon DM, Liu J, Zhang H, Torres ME, Best RG (February 2013). “Maternal obesity, folate intake, and neural tube defects in offspring”. Birth Defects Research Part A: Clinical and Molecular Teratology 97 (2): 115–122. doi:10.1002/bdra.23113. PMID 23404872.
- Jump up^ Brambilla G, Mattioli F, Martelli A (2009). “Genotoxic and carcinogenic effects of antipsychotics and antidepressants”. Toxicology 261 (3): 77–88.doi:10.1016/j.tox.2009.04.056. PMID 19410629.
- Jump up^ “4.2.1 Antipsychotic drugs”. British National Formulary (57th ed.). London and Chicago: Pharmaceutical Press. March 2009. pp. 221–31. ISBN 978-0-85711-065-7.
Withdrawal of antipsychotic drugs after long-term therapy should always be gradual and closely monitored to avoid the risk of acute withdrawal syndromes or rapid relapse.
- Jump up^ “Harm Reduction Guide To Coming Off Psychiatric Drugs & Withdrawal”. Icarus Project. April 23, 2008.
- Jump up^ Kim DR, Staab JP (2005). “Quetiapine Discontinuation Syndrome”. American Journal of Psychiatry 162 (5): 1020. doi:10.1176/appi.ajp.162.5.1020. PMID 15863814.
- Jump up^ Michaelides C, Thakore-James M, Durso R (2005). “Reversible withdrawal dyskinesia associated with quetiapine”. Movement Disorders 20 (6): 769–70.doi:10.1002/mds.20427. PMID 15747370.
- Jump up^ Chouinard G, Jones BD (1980). “Neuroleptic-induced supersensitivity psychosis: Clinical and pharmacologic characteristics”. The American Journal of Psychiatry 137 (1): 16–21.PMID 6101522.
- Jump up^ Miller R, Chouinard G (1993). “Loss of striatal cholinergic neurons as a basis for tardive and L-dopa-induced dyskinesias, neuroleptic-induced supersensitivity psychosis and refractory schizophrenia”. Biological Psychiatry 34 (10): 713–38. doi:10.1016/0006-3223(93)90044-E. PMID 7904833.
- Jump up^ Chouinard G, Jones BD, Annable L (1978). “Neuroleptic-induced supersensitivity psychosis”. The American Journal of Psychiatry 135 (11): 1409–10. PMID 30291.
- Jump up^ Seeman P, Weinshenker D, Quirion R, Srivastava LK, Bhardwaj SK, Grandy DK, Premont RT, Sotnikova TD, Boksa P, El-Ghundi M, O’dowd BF, George SR, Perreault ML, Männistö PT, Robinson S, Palmiter RD, Tallerico T (2005). “Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis”. Proceedings of the National Academy of Sciences 102 (9): 3513–8. Bibcode:2005PNAS..102.3513S.doi:10.1073/pnas.0409766102. JSTOR 3374957. PMC 548961. PMID 15716360.
- Jump up^ Moncrieff J (2006). “Does antipsychotic withdrawal provoke psychosis? Review of the literature on rapid onset psychosis (supersensitivity psychosis) and withdrawal-related relapse”. Acta Psychiatrica Scandinavica 114 (1): 3–13. doi:10.1111/j.1600-0447.2006.00787.x. PMID 16774655.
- ^ Jump up to:a b “Symbyax (Olanzapine and fluoxetine) drug overdose and contraindication information”. RxList: The Internet Drug Index. WebMD. 2007. Retrieved 2007-12-03.
- Jump up^ Wang JS, Zhu HJ, Markowitz JS, Donovan JL, DeVane CL (September 2006). “Evaluation of antipsychotic drugs as inhibitors of multidrug resistance transporter P-glycoprotein”. Psychopharmacology (Berl) 187 (4): 415–423. doi:10.1007/s00213-006-0437-9. PMID 16810505.
- Jump up^ Moons T, de Roo M, Claes S, Dom G (August 2011). “Relationship between P-glycoprotein and second-generation antipsychotics”. Pharmacogenomics 12 (8): 1193–1211. doi:10.2217/pgs.11.55. PMID 21843066.
- Jump up^ Roth, BL; Driscol, J (12 January 2011). “PDSP Ki Database”. Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 26 November 2013.
- ^ Jump up to:a b c d e Brunton, L; Chabner, B; Knollman, B (2010). Goodman and Gilman’s The Pharmacological Basis of Therapeutics (12th ed.). New York: McGraw-Hill Professional.ISBN 978-0-07-162442-8.
- ^ Jump up to:a b Weston-Green K, Huang XF, Deng C (10 October 2013). “Second Generation Antipsychotic-Induced Type 2 Diabetes: A Role for the Muscarinic M3 Receptor”. CNS Drugs 27: 1069–1080. doi:10.1007/s40263-013-0115-5. PMID 24114586. Retrieved15 February 2014.
- Jump up^ Johnson DE, Yamazaki H, Ward KM, Schmidt AW, Lebel WS, Treadway JL, Gibbs EM, Zawalich WS, Rollema H (2005). “Inhibitory Effects of Antipsychotics on Carbachol-Enhanced Insulin Secretion from Perifused Rat Islets: Role of Muscarinic Antagonism in Antipsychotic-Induced Diabetes and Hyperglycemia”. Diabetes 54 (5): 1552–8.doi:10.2337/diabetes.54.5.1552. PMID 15855345.
- Jump up^ Silvestre JS, Prous J (2005). “Research on adverse drug events. I. Muscarinic M3 receptor binding affinity could predict the risk of antipsychotics to induce type 2 diabetes”.Methods and Findings in Experimental and Clinical Pharmacology 27 (5): 289–304.doi:10.1358/mf.2005.27.5.908643. PMID 16082416.
- Jump up^ “olanzapine”. NCI Drug Dictionary. National Cancer Institute.
- Jump up^ Lemke TL, Williams DA (2009) Foye’s Medicinal Chemistry, 6th edition. Wolters Kluwer: New Delhi. ISBN 978-81-89960-30-8.
- Jump up^ “Medication and Weight Control”
- Jump up^ “NDA 21-520″ (PDF). Food and Drug Administration. 2003-12-24. Retrieved2009-09-06.
- Jump up^ “NDA 20-592 / S-019″ (PDF). Food and Drug Administration. 2004-01-14. Retrieved2009-09-06.
- Jump up^ Pillarella J, Higashi A, Alexander GC, Conti R (2012). “Trends in the use of atypical antipsychotics for the treatment of bipolar disorder in the United States, 1998-2009″.Psychiatric Services 63 (1): 83–86. doi:10.1176/appi.ps.201100092. PMID 22227765.
- Jump up^ Forbes.com[dead link]
- Jump up^ treatment resistant depression defined as major depressive disorder in adult patients who do not respond to two separate trials of different antidepressants of adequate dose and duration in the current episode
- Jump up^ “NDA 20-592″ (PDF). Food and Drug Administration. 1996-09-06. Retrieved2009-09-06.
- ^ Jump up to:a b c d “Eli Lilly and Company Agrees to Pay $1.415 Billion to Resolve Allegations of Off-label Promotion of Zyprexa”. U.S. Justice Department. 2009-01-15. Retrieved2012-07-09.
- Jump up^ Berenson, Alex (January 4, 2007). “Mother Wonders if Psychosis Drug Helped Kill Son”. The New York Times. Retrieved May 21, 2013.
- Jump up^ MSN.com Lilly settles Zyprexa suit for $1.42 billion.
- Jump up^ Berenson, Alex (December 18, 2006). “Drug Files Show Maker Promoted Unapproved Use”. The New York Times. Retrieved May 21, 2013.
- Jump up^ Berenson, Alex (December 17, 2006). “Eli Lilly Said to Play Down Risk of Top Pill”.The New York Times. Retrieved May 21, 2013.
- ^ Jump up to:a b c L-Z\In re Zyprexa\Documents Leak\Injunction Memo & Order\FINAL INJUNCTION MEMO 2.13.07.wpd
- Jump up^ EFF.org
- Jump up^ Press Releases: January, 2007 | Electronic Frontier Foundation
- ^ Jump up to:a b Eli Lilly was Concerned by Zyprexa Side-Effects from 1998,[dead link] The Times (London), January 23, 2007
- Jump up^ Navari RM, Einhorn LH, Loehrer PJ, Passik SD, Vinson J, McClean J, Chowhan N, Hanna NH, Johnson CS (2007). “A phase II trial of olanzapine, dexamethasone, and palonosetron for the prevention of chemotherapy-induced nausea and vomiting: A Hoosier oncology group study”. Supportive Care in Cancer 15 (11): 1285–91. doi:10.1007/s00520-007-0248-5. PMID 17375339.
- Jump up^ McGlashan TH, Zipursky RB, Perkins D, Addington J, Miller TJ, Woods SW, Hawkins KA, Hoffman R, Lindborg S, Tohen M, Breier A (2003). “The PRIME North America randomized double-blind clinical trial of olanzapine versus placebo in patients at risk of being prodromally symptomatic for psychosis”. Schizophrenia Research 61 (1): 7–18.doi:10.1016/S0920-9964(02)00439-5. PMID 12648731.
- Jump up^ McGlashan TH, Zipursky RB, Perkins D, Addington J, Miller T, Woods SW, Hawkins KA, Hoffman RE, Preda A, Epstein I, Addington D, Lindborg S, Trzaskoma Q, Tohen M, Breier A (2006). “Randomized, Double-Blind Trial of Olanzapine Versus Placebo in Patients Prodromally Symptomatic for Psychosis”. American Journal of Psychiatry 163 (5): 790–9.
External links
- Zyprexa.com – official Zyprexa brand website from Eli Lilly and Company
- Zyprexa package insert
- Berenson, Alex (December 17, 2006). “Lilly Settles With 18,000 Over Zyprexa”. New York Times.

 COCK WILL TEACH YOU NMR
COCK WILL TEACH YOU NMR COCK SAYS MOM CAN TEACH YOU NMR
COCK SAYS MOM CAN TEACH YOU NMR
 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..FOR BLOG HOME CLICK HERE![]() amcrasto@gmail.com
amcrasto@gmail.com
Filed under: Uncategorized Tagged: antipsychotic drugs, atypical antipsychotics, eli lilly, Food and Drug Administration, OLANZEPINE, treatment of schizophrenia


 amcrasto@gmail.com
amcrasto@gmail.com
















