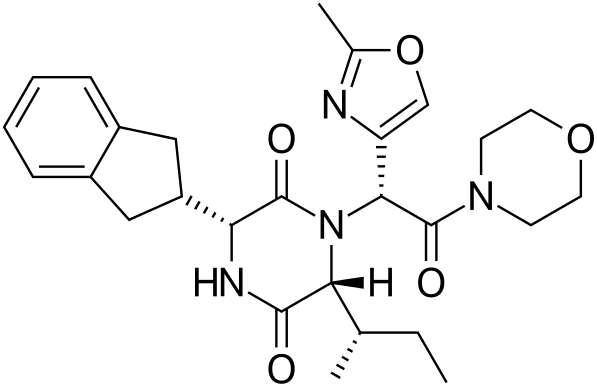
Retosiban, GSK221149A
820957-38-8
MW 494.5827, MF C27 H34 N4 O5
Oxytocin antagonist
Threatened pre-term labour
PHASE 3 GSK

UNII-GIE06H28OX, GSK 221149A, 820957-38-8,
(3R,6R)-6-((S)-sec-butyl)-3-(2,3-dihydro-1H-inden-2-yl)-1-((R)-1-(2-methyloxazol-4-yl)-2-morpholino-2-oxoethyl)piperazine-2,5-dione
3(R)-(2,3-Dihydro-1H-inden-2-yl)-1-[1(R)-(2-methyloxazol-4-yl)-2-(4-morpholinyl)-2-oxoethyl]-6(R)-[1(S)-methylpropyl]piperazine-2,5-dione
(3R.6R)-3-(2,3-dihvdro-1 H-inden-2-v0-1 -\( R)-1 -(2-methyl-1 ,3-oxazol-4- yl)-2-(4-morpholinyl)-2-oxoethyll-6-r(1S -1-methylpropyn-2.5- piperazinedione
2,5-Piperazinedione, 3-(2,3-dihydro-1H-inden-2-yl)-1-[(1R)-1-(2-methyl-4-oxazolyl)-2-(4-morpholinyl)-2-oxoethyl]-6-[(1S)-1-methylpropyl]-, (3R,6R)-
Morpholine, 4-[(2R)-[(3R,6R)-3-(2,3-dihydro-1H-inden-2-yl)-6-[(1S)-1-methylpropyl]-2,5-dioxo-1-piperazinyl](2-methyl-4-oxazolyl)acetyl]-
Retosiban (GSK-221,149-A)[1][2] is an oral drug which acts as a selective, sub-nanomolar (Ki = 0.65 nM) oxytocin receptor antagonist with >1400-fold selectivity[3] over the related vasopressin receptors and is being developed by GlaxoSmithKline for the treatment of preterm labour.[4][5]
Retosibanis an oxytocin (OT) antagonist in phase III clinical trials at GlaxoSmithKline for the prevention of preterm labor. OT antagonism is widely known to inhibit spontaneous uterine contractions.
Retosiban is a diketopiperazine nonpeptide compound with high potency and selectivity for the OT receptor over vasopressin receptors.
This candidate has been shown to block oxytocin-induced uterine contractions when administered intravenously and to exhibit oral activity
 ATOSIBAN
ATOSIBANSee also,(b) Coomarasamy, A.; Knox, E. M.; Gee, H.; Khan, K. S.Oxytocin antagonists for tocolysis in preterm labour—a systematic review Med. Sci. Monit. 2002, 8, RA268– RA273)
 RETOSIBAN 106
RETOSIBAN 106
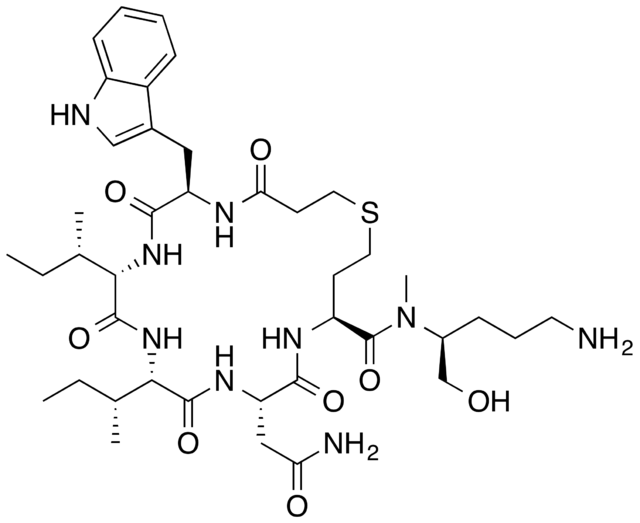 BARUSIBAN
BARUSIBAN
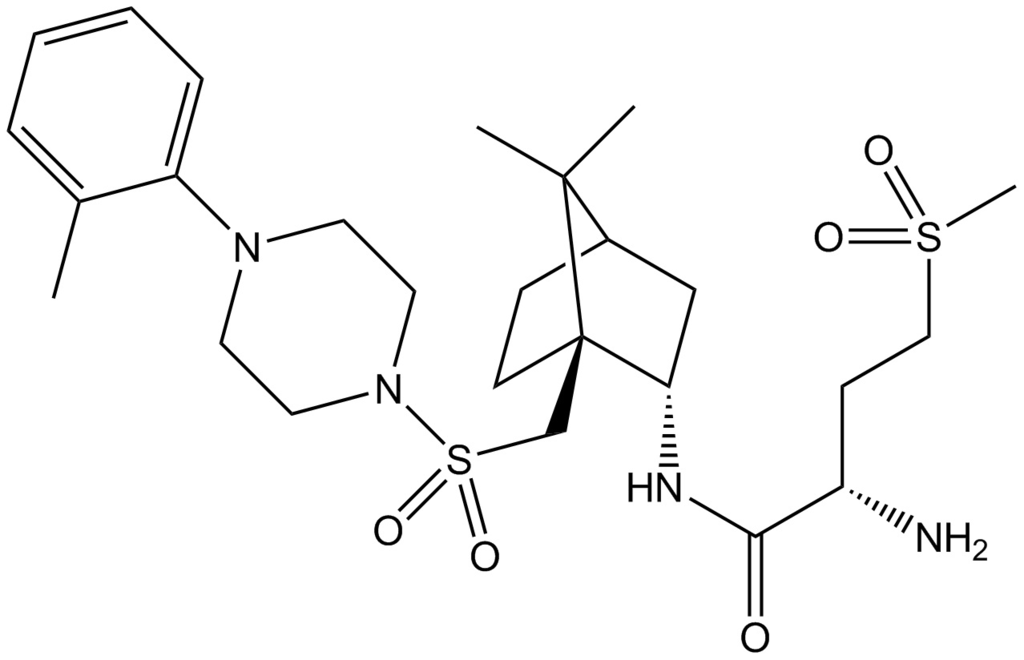 L-368899
L-368899
 L-371257
L-371257
PAPER
Pyridyl-2,5-diketopiperazines as potent, selective, and orally bioavailable oxytocin antagonists: Synthesis, pharmacokinetics, and in vivo potency
J Med Chem 2012, 55(2): 783
http://pubs.acs.org/doi/abs/10.1021/jm201287w
PAPER
The discovery of GSK221149A: A potent and selective oxytocin antagonist
Bioorg Med Chem Lett 2008, 18(1): 90
http://www.sciencedirect.com/science/article/pii/S0960894X07013170
Reagents and conditions: (a) triethylamine, MeOH; (b) H2, Pd/C, ethanol/acetic acid; (c) carbonyl diimidazole, CH2Cl2 3 h then acetone/2 N HCl; (d) benzotriazol-1-yloxytripyrrolidinophosphonium hexafluorophosphate, dichloromethane 1 h then morpholine.
GSK221149A and other tertiary amides were prepared in four steps via the Ugi reaction as outlined in Scheme . A 2:1 mixture of diastereoisomers 24 was formed with the desirable (R)-diastereoisomer being the minor product. Hydrogenation of crude 24 furnished the cyclised phenol 25, again enriched with the undesirable (S)-diastereoisomer.
Activation of the mixture 25 with carbonyl diimidazole followed by the addition of 2 N HCl promoted epimerisation at the exocyclic position and yielded the acids 26 with the required (R)-diastereoisomer as the major product.
Acid activation with benzotriazol-1-yloxytripyrrolidinophosphonium hexafluorophosphate followed by the addition of morpholine and subsequent column chromatography yielded homo-chiral GSK221149A.
PATENT
WO 2005000840
http://www.google.co.in/patents/EP1641787A1?cl=en
Example 3
(3R.6R)-3-(2,3-dihvdro-1 H-inden-2-v0-1 -\( R)-1 -(2-methyl-1 ,3-oxazol-4- yl)-2-(4-morpholinyl)-2-oxoethyll-6-r(1S -1-methylpropyn-2.5- piperazinedione ( 2R)-[(benzyloxycarbonyl)amino](2,3-dihydro-1 H-inden-2-yl)ethanoic acid (35.84g, 0.110mol) in a 500mL round bottomed flask was treated with 2,2,2-trifluoroethanol (165mL) followed by methanol (55ml) and triethylamine (11.13g, 15.33mL, 0.110mmol) the slurry was stirred for 3.5hrs until dissolution was observed. The solution was then added to (D)- allo Isoleucine methyl ester hydrochloride (20g, .110mol) in a separate flask. The slurry was stirred until dissolution was observed. 2-methyl-4- formyloxazole (12.24g, 0.110mmol) was then added followed by 2- benzyloxyphenylisocynanide (23.04g, 0.110mmol). The dark brown reaction mixture was then stirred at 20-25°C for 24hrs. The solution was then concentrated to a volume of ca. 130mL by distillation at reduced pressure.
The solution was the diluted with dichloromethane (200mL) and washed with water (2 x 200mL). The organic phase was then diluted with N-methyl pyrrolidinone (460mL) was and the dichloromethane removed by stirring at 40°C under vacuum for 2hrs. Acetic acid 46mL) was then added followed by palladium on carbon catalyst (69. Og of 10% Pd wt, 57% water, Johnson Matthey type 87L) and the mixture hydrogenated under balloon pressure of hydrogen with rapid stirring for 2hrs. The reaction mixture was then filtered, washed through with ethyl acetate (960mL) and washed with 3%w/v aq sodium chloride solution (960mL). The biphasic mixture was filtered and the organic phase separated and washed with 3%w/v aq sodium chloride solution (2 x 960mL). The organic solution was then diluted with ethyl acetate (200mL) and concentrated by distillation at atmospheric pressure by distilling out 385mL of solvent. The concentrated solution at 20-25°C was treated with 1 ,1′-carbonyldiimidazoIe (21.46g, 0.132mol) and stirred at 20-25°C for 1 hr then treated with water (290mL) and stirred rapidly at 20-25°C for 24hr. The mixture was allowed to settle and the ethyl acetate layer separated and discarded. The aqueous phase was washed with ethyl acetate (290mL) and the mixture allowed to settle and the aqueous phase was separated and acidified to pH 1-2 by the addition of concentrated hydrochloric acid (18mL).
The aqueous phase was then extracted into ethyl acetate (290mL and then 145mL). The combined ethyl acetate solution was then concentrated by distillation at atmospheric pressure to a volume of ca. 93mL. This solution was then diluted with tetrahydrofuran (62mL) and treated with triethylamine (11.02g, 15.20mL, 0.109mol) and cooled to -78°C. The solution was then treated with trimethylacetyl chloride (4.81 g, 4.92mL, 39.90mmol) and stirred at – 78°C for 7hr. The reaction mixture was then treated with a solution of morpholine (15.82g, 15.83mL, 0.181 mol) in tetrahydrofuran (23mL) and stirred at -78°C for 1hr 20mins before being allowed to warm to 20-25°C. The solution was then diluted with ethyl acetate (76mL) and washed with saturated aqueous sodium bicarbonate solution (2 x 153mL) followed by water (153mL). The organic solution was then diluted with ethyl acetate (54mL) and distilled down to a volume of 69mL at atmospheric pressure. The solution was then cooled to 20-25°C at which point crystallisation of the title compound occurred. The slurry of was then cooled further to 0°C before the title compound was isolated by filtration and sucked dry. Yield 8.92g.
SYN WILL BE UPDATED.. ……………KEEP WATCHING
References
- 1 Liddle J, Allen MJ, Borthwick AD, Brooks DP, Davies DE, Edwards RM, Exall AM, Hamlett C, Irving WR, Mason, AM, McCafferty GP, Nerozzi F, Peace S, Philp J, Pollard D, Pullen MA, Shabbir SS, Sollis SL, Westfall TD, Woollard PM, Wu C, Hickey DM (January 2008). “The discovery of GSK221149A: A potent and selective oxytocin antagonist”. Bioorganic & Medicinal Chemistry Letters 18 (1): 90–94. doi:10.1016/j.bmcl.2007.11.008. PMID 18032036.
- 2
- Borthwick, A. D.; Liddle, J. (January 2013). “Retosiban and Epelsiban: Potent and Selective Orally available Oxytocin Antagonists”. In Domling, A. Methods and Principles in Medicinal Chemistry: Protein-Protein Interactions in Drug Discovery. Weinheim: Wiley-VCH. pp. 225–256. ISBN 978-3-527-33107-9.
- 3
- McCafferty GP, Pullen MA, Wu C, Edwards RM, Allen M.J, Woollard PM, Borthwick AD, Liddle J, Hickey DM, Brooks DP, Westfall TD (March 2007). “Use of a novel and highly selective oxytocin receptor antagonist to characterize uterine contractions in the rat”. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology 293: R299–R305. doi:10.1152/ajpregu.00057.2007. PMID 17395790.
- 4
- USAN Council (2007). “Statement on a Nonproprietary Name Adopted by the USAN Council” (PDF).
- 5 Borthwick AD, Liddle J (July 2011). “The Design of Orally Bioavailable 2,5-Diketopiperazine Oxytocin Antagonists: From Concept to Clinical Candidate for Premature Labour”. Medicinal Research Reviews 31 (4): 576–604. doi:10.1002/med.20193. PMID 20027670.
…………..
OTHER INFO
http://pubs.acs.org/doi/abs/10.1021/jm201287w

A six-stage stereoselective synthesis of indanyl-7-(3′-pyridyl)-(3R,6R,7R)-2,5-diketopiperazines oxytocin antagonists from indene is described. SAR studies involving mono- and disubstitution in the 3′-pyridyl ring and variation of the 3-isobutyl group gave potent compounds (pKi > 9.0) with good aqueous solubility. Evaluation of the pharmacokinetic profile in the rat, dog, and cynomolgus monkey of those derivatives with low cynomolgus monkey and human intrinsic clearance gave 2′,6′-dimethyl-3′-pyridyl R-sec-butyl morpholine amide Epelsiban (69), a highly potent oxytocin antagonist (pKi = 9.9) with >31000-fold selectivity over all three human vasopressin receptors hV1aR, hV2R, and hV1bR, with no significant P450 inhibition. Epelsiban has low levels of intrinsic clearance against the microsomes of four species, good bioavailability (55%) and comparable potency to atosiban in the rat, but is 100-fold more potent than the latter in vitro and was negative in the genotoxicity screens with a satisfactory oral safety profile in female rats.
EPELSIBAN
(3R,6R)-3-(2,3-Dihydro-1H-inden-2-yl)-1-[(1R)-1-(2,6-dimethyl-3-pyridinyl)-2-(4-morpholinyl)-2-oxoethyl]-6-[(1S)-1-methylpropyl]-2,5-piperazinedione (69)
…………..
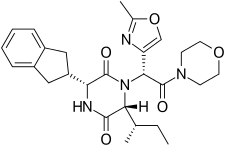 |
|
| Systematic (IUPAC) name | |
|---|---|
| (3R,6R)-6-[(2S)-butan-2-yl]-3-(2,3-dihydro-1H-inden-2-yl)-1-[(1R)-1-(2-methyl-1,3-oxazol-4-yl)-2-(morpholin-4-yl)-2-oxoethyl]piperazine-2,5-dione | |
| Clinical data | |
| Legal status |
|
| Identifiers | |
| CAS number | 820957-38-8 |
| ATC code | None |
| PubChem | CID 96025669 |
| ChemSpider | 23323798 |
| UNII | GIE06H28OX |
| KEGG | D08986 |
| Synonyms | GSK-221,149-A |
| Chemical data | |
| Formula | C27H34N4O5 |
| Molecular mass | 494.58 g/mol |
Filed under: Phase3 drugs, Uncategorized Tagged: gsk, GSK221149A, PHASE 3, retosiban

