FAVIPIRAVIR
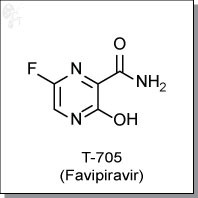
| Chemical Formula: | C5H4FN3O2 | |
| CAS #: | 259793-96-9 | |
| Molecular Weight: | 157.1 | |
ANTI-INFLUENZA COMPOUND |
||
| clinical trials | http://clinicaltrials.gov/search/intervention=Favipiravir | |
| Chemical Name: | 6-fluoro-3-hydroxy-2-pyrazinecarboxamide | |
| Synonyms: | T-705, T705, Favipiravir |
- Furuta, Y.; Takahashi, K.; Shiraki, K.; Sakamoto, K.; Smee, D. F.; Barnard, D. L.; Gowen, B. B.; Julander, J. G.; Morrey, J. D. (2009). “T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections”. Antiviral Research 82 (3): 95–102. doi:10.1016/j.antiviral.2009.02.198. PMID 19428599. edit
![]()
- WO 2000010569
- WO 2008099874
- WO 201009504
- WO 2010104170
- WO 2012063931


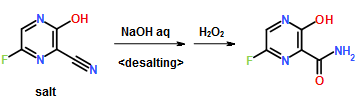
……………………………………………………………………………………
Influenza virus is a central virus of the cold syndrome, which has attacked human being periodically to cause many deaths amounting to tens millions. Although the number of deaths shows a tendency of decrease in the recent years owing to the improvement in hygienic and nutritive conditions, the prevalence of influenza is repeated every year, and it is apprehended that a new virus may appear to cause a wider prevalence.
For prevention of influenza virus, vaccine is used widely, in addition to which low molecular weight substances such as Amantadine and Ribavirin are also used
……………………………….

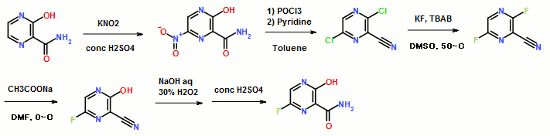
Synthesis of Favipiravir
ZHANG Tao1, KONG Lingjin1, LI Zongtao1,YUAN Hongyu1, XU Wenfang2*
(1. Shandong Qidu PharmaceuticalCo., Ltd., Linzi 255400; 2. School of Pharmacy, Shandong University, Jinan250012)
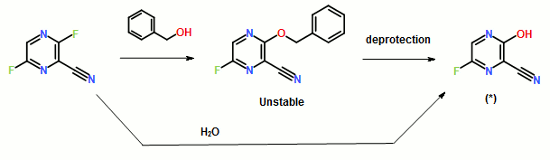
ABSTRACT: Favipiravir was synthesized from3-amino-2-pyrazinecarboxylic acid by esterification, bromination with NBS,diazotization and amination to give 6-bromo-3-hydroxypyrazine-2-carboxamide,which was subjected to chlorination with POCl3, fluorination with KF, andhydrolysis with an overall yield of about 22%.
………………………………..
US6787544

| subs G1 | G2 | G3 | G4 | R2 |
| compd 32 N | CH | C—CF3 | N | H |
…………………
EP2192117

Example 1-1
To a 17.5 ml N,N-dimethylformamide solution of 5.0 g of 3,6-difluoro-2-pyrazinecarbonitrile, a 3.8 ml water solution of 7.83 g of potassium acetate was added dropwise at 25 to 35° C., and the solution was stirred at the same temperature for 2 hours. 0.38 ml of ammonia water was added to the reaction mixture, and then 15 ml of water and 0.38 g of active carbon were added. The insolubles were filtered off and the filter cake was washed with 11 ml of water. The filtrate and the washing were joined, the pH of this solution was adjusted to 9.4 with ammonia water, and 15 ml of acetone and 7.5 ml of toluene were added. Then 7.71 g of dicyclohexylamine was added dropwise and the solution was stirred at 20 to 30° C. for 45 minutes. Then 15 ml of water was added dropwise, the solution was cooled to 10° C., and the precipitate was filtered and collected to give 9.44 g of dicyclohexylamine salt of 6-fluoro-3-hydroxy-2-pyradinecarbonitrile as a lightly yellowish white solid product.
1H-NMR (DMSO-d6) δ values: 1.00-1.36 (10H, m), 1.56-1.67 (2H, m), 1.67-1.81 (4H, m), 1.91-2.07 (4H, m), 3.01-3.18 (2H, m), 8.03-8.06 (1H, m), 8.18-8.89 (1H, broad)
Example 1-2
4.11 ml of acetic acid was added at 5 to 15° C. to a 17.5 ml N,N-dimethylformamide solution of 5.0 g of 3,6-difluoro-2-pyrazinecarbonitrile. Then 7.27 g of triethylamine was added dropwise and the solution was stirred for 2 hours. 3.8 ml of water and 0.38 ml of ammonia water were added to the reaction mixture, and then 15 ml of water and 0.38 g of active carbon were added. The insolubles were filtered off and the filter cake was washed with 11 ml of water. The filtrate and the washing were joined, the pH of the joined solution was adjusted to 9.2 with ammonia water, and 15 ml of acetone and 7.5 ml of toluene were added to the solution, followed by dropwise addition of 7.71 g of dicyclohexylamine. Then 15 ml of water was added dropwise, the solution was cooled to 5° C., and the precipitate was filtered and collected to give 9.68 g of dicyclohexylamine salt of 6-fluoro-3-hydroxy-2-pyrazinecarbonitrile as a slightly yellowish white solid product.
Examples 2 to 5
The compounds shown in Table 1 were obtained in the same way as in Example 1-1.
| TABLE 1 | |||
 |
|||
| Example No. | Organic amine | Example No. | Organic amine |
| 2 | Dipropylamine | 4 | Dibenzylamine |
| 3 | Dibutylamine | 5 | N-benzylmethylamine |
Dipropylamine salt of 6-fluoro-3-hydroxy-2-pyrazinecarbonitrile
1H-NMR (DMSO-d6) 6 values: 0.39 (6H, t, J=7.5 Hz), 1.10 (4H, sex, J=7.5 Hz), 2.30-2.38 (4H, m), 7.54 (1H, d, J=8.3 Hz)
Dibutylamine salt of 6-fluoro-3-hydroxy-2-pyrazinecarbonitrile
1H-NMR (DMSO-d6) 6 values: 0.36 (6H, t, J=7.3 Hz), 0.81 (4H, sex, J=7.3 Hz), 0.99-1.10 (4H, m), 2.32-2.41 (4H, m), 7.53 (1H, d, J=8.3 Hz)
Dibenzylamine salt of 6-fluoro-3-hydroxy-2-pyrazinecarbonitrile
1H-NMR (DMSO-d6) δ values: 4.17 (4H, s), 7.34-7.56 (10H, m), 8.07 (1H, d, J=8.3 Hz)
N-benzylmethylamine salt of 6-fluoro-3-hydroxy-2-pyrazinecarbonitrile
1H-NMR (DMSO-d6) δ values: 2.57 (3H, s), 4.14 (2H, s), 7.37-7.53 (5H, m), 8.02-8.08 (1H, m)
Preparation Example 1
300 ml of toluene was added to a 600 ml water solution of 37.5 g of sodium hydroxide. Then 150 g of dicyclohexylamine salt of 6-fluoro-3-hydroxy-2-pyrazinecarbonitrile was added at 15 to 25° C. and the solution was stirred at the same temperature for 30 minutes. The water layer was separated and washed with toluene, and then 150 ml of water was added, followed by dropwise addition of 106 g of a 30% hydrogen peroxide solution at 15 to 30° C. and one-hour stirring at 20 to 30° C. Then 39 ml of hydrochloric acid was added, the seed crystals were added at 40 to 50° C., and 39 ml of hydrochloric acid was further added dropwise at the same temperature. The solution was cooled to 10° C. the precipitate was filtered and collected to give 65.6 g of 6-fluoro-3-hydroxy-2-pyrazinecarboxamide as a slightly yellowish white solid.
1H-NMR (DMSO-d6) δ values: 8.50 (1H, s), 8.51 (1H, d, J=7.8 Hz), 8.75 (1H, s), 13.41 (1H, s)
………………….
jan 2014
First patient enrolled in the North American Phase 3 clinical trials for investigational flu treatment drug
Eur J Med Chem. 2013 Apr;62:534-44. doi: 10.1016/j.ejmech.2013.01.015. Epub 2013 Jan 29.
| US3631036 * | Nov 4, 1969 | Dec 28, 1971 | American Home Prod | 5-amino-2 6-substituted-7h-pyrrolo(2 3-d) pyrimidines and related compounds |
| US3745161 * | Apr 20, 1970 | Jul 10, 1973 | Merck & Co Inc | Phenyl-hydroxy-pyrazine carboxylic acids and derivatives |
| US4404203 * | May 14, 1981 | Sep 13, 1983 | Warner-Lambert Company | Substituted 6-phenyl-3(2H)-pyridazinones useful as cardiotonic agents |
| US4545810 * | Mar 25, 1983 | Oct 8, 1985 | Sds Biotech Corporation | Herbicidal and plant growth regulant diphenylpyridazinones |
| US4565814 * | Jan 18, 1984 | Jan 21, 1986 | Sanofi | Pyridazine derivatives having a psychotropic action and compositions |
| US4661145 * | Sep 20, 1984 | Apr 28, 1987 | Rohm And Haas Company | Plant growth regulating 1-aryl-1,4-dihydro-4-oxo(thio)-pyridazines |
| US5420130 | May 16, 1994 | May 30, 1995 | Synthelabo | 2-aminopyrazine-5-carboxamide derivatives, their preparation and their application in therapeutics |
| US5459142 * | Aug 23, 1993 | Oct 17, 1995 | Otsuka Pharmaceutical Co., Ltd. | Pyrazinyl and piperazinyl substituted pyrazine compounds |
| US5597823 | Jun 5, 1995 | Jan 28, 1997 | Abbott Laboratories | Tricyclic substituted hexahydrobenz [e]isoindole alpha-1 adrenergic antagonists |
| US6159980 * | Sep 15, 1997 | Dec 12, 2000 | Dupont Pharmaceuticals Company | Pyrazinones and triazinones and their derivatives thereof |
| EP0023358A1 * | Jul 28, 1980 | Feb 4, 1981 | Rohm And Haas Company | Process for the preparation of pyridazine derivatives |
| GB1198688A | Title not available | |||
| HU9401512A | Title not available | |||
| JPH09216883A * | Title not available | |||
| JPS5620576A | Title not available |

Want to know everything on vir series
click
http://drugsynthesisint.blogspot.in/p/vir-series-hep-c-virus-22.html
AND
http://medcheminternational.blogspot.in/p/vir-series-hep-c-virus.html
Filed under: Uncategorized


