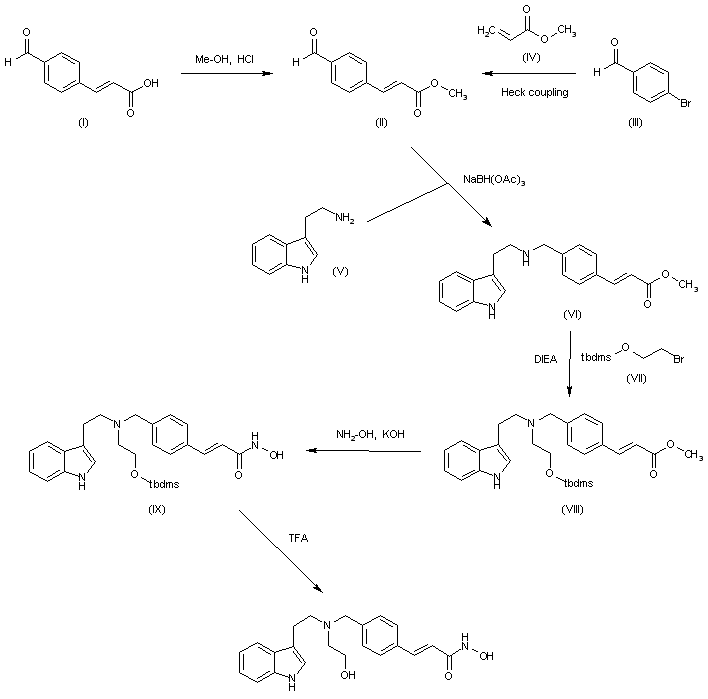
C22H25N3O3
Exact Mass: 379.18959
Molecular Weight: 379.45
Clik here to view.

Reversible acetylation of histones is a major regulator of gene expression that acts by altering accessibility of transcription factors to DNA. In normal cells, histone deacetylase (HDA) and histone acetyltrasferase together control the level of acetylation of histones to maintain a balance. Inhibition of HDA results in the accumulation of hyperacetylated histones, which results in a variety of cellular responses.
Inhibitors of HDA have been studied for their therapeutic effects on cancer cells. For example, butyric acid and its derivatives, including sodium phenylbutyrate, have been reported to induce apoptosis in vitro in human colon carcinoma, leukemia and retinoblastoma cell lines. However, butyric acid and its derivatives are not useful pharmacological agents because they tend to be metabolized rapidly and have a very short half-life in vivo. Other inhibitors of HDA that have been widely studied for their anti-cancer activities are trichostatin A and trapoxin. Trichostatin A is an antifungal and antibiotic and is a reversible inhibitor of mammalian HDA. Trapoxin is a cyclic tetrapeptide, which is an irreversible inhibitor of mammalian HDA.
Although trichostatin and trapoxin have been studied for their anti-cancer activities, the in vivo instability of the compounds makes them less suitable as anti-cancer drugs. There remains a need for an active compound that is suitable for treating tumors, including cancerous tumors, that is highly efficacious and stable
Clik here to view.
|
References |
1: Wang H, Cheng F, Woan K, Sahakian E, Merino O, Rock-Klotz J, Vicente-Suarez I, Pinilla-Ibarz J, Wright KL, Seto E, Bhalla K, Villagra A, Sotomayor EM. Histone deacetylase inhibitor LAQ824 augments inflammatory responses in macrophages through transcriptional regulation of IL-10. J Immunol. 2011 Apr 1;186(7):3986-96. doi: 10.4049/jimmunol.1001101. Epub 2011 Mar 2. PubMed PMID: 21368229.
2: Schwarz K, Romanski A, Puccetti E, Wietbrauk S, Vogel A, Keller M, Scott JW, Serve H, Bug G. The deacetylase inhibitor LAQ824 induces notch signalling in haematopoietic progenitor cells. Leuk Res. 2011 Jan;35(1):119-25. doi: 10.1016/j.leukres.2010.06.024. Epub 2010 Jul 31. PubMed PMID: 20674020.
3: Cho YS, Whitehead L, Li J, Chen CH, Jiang L, Vögtle M, Francotte E, Richert P, Wagner T, Traebert M, Lu Q, Cao X, Dumotier B, Fejzo J, Rajan S, Wang P, Yan-Neale Y, Shao W, Atadja P, Shultz M. Conformational refinement of hydroxamate-based histone deacetylase inhibitors and exploration of 3-piperidin-3-ylindole analogues of dacinostat (LAQ824). J Med Chem. 2010 Apr 8;53(7):2952-63. doi: 10.1021/jm100007m. PubMed PMID: 20205394.
4: Vo DD, Prins RM, Begley JL, Donahue TR, Morris LF, Bruhn KW, de la Rocha P, Yang MY, Mok S, Garban HJ, Craft N, Economou JS, Marincola FM, Wang E, Ribas A. Enhanced antitumor activity induced by adoptive T-cell transfer and adjunctive use of the histone deacetylase inhibitor LAQ824. Cancer Res. 2009 Nov 15;69(22):8693-9. doi: 10.1158/0008-5472.CAN-09-1456. Epub 2009 Oct 27. PubMed PMID: 19861533; PubMed Central PMCID: PMC2779578.
5: Ellis L, Bots M, Lindemann RK, Bolden JE, Newbold A, Cluse LA, Scott CL, Strasser A, Atadja P, Lowe SW, Johnstone RW. The histone deacetylase inhibitors LAQ824 and LBH589 do not require death receptor signaling or a functional apoptosome to mediate tumor cell death or therapeutic efficacy. Blood. 2009 Jul 9;114(2):380-93. doi: 10.1182/blood-2008-10-182758. Epub 2009 Apr 21. PubMed PMID: 19383971.
6: de Bono JS, Kristeleit R, Tolcher A, Fong P, Pacey S, Karavasilis V, Mita M, Shaw H, Workman P, Kaye S, Rowinsky EK, Aherne W, Atadja P, Scott JW, Patnaik A. Phase I pharmacokinetic and pharmacodynamic study of LAQ824, a hydroxamate histone deacetylase inhibitor with a heat shock protein-90 inhibitory profile, in patients with advanced solid tumors. Clin Cancer Res. 2008 Oct 15;14(20):6663-73. doi: 10.1158/1078-0432.CCR-08-0376. PubMed PMID: 18927309.
7: Chung YL, Troy H, Kristeleit R, Aherne W, Jackson LE, Atadja P, Griffiths JR, Judson IR, Workman P, Leach MO, Beloueche-Babari M. Noninvasive magnetic resonance spectroscopic pharmacodynamic markers of a novel histone deacetylase inhibitor, LAQ824, in human colon carcinoma cells and xenografts. Neoplasia. 2008 Apr;10(4):303-13. PubMed PMID: 18392140; PubMed Central PMCID: PMC2288545.
8: Cuneo KC, Fu A, Osusky K, Huamani J, Hallahan DE, Geng L. Histone deacetylase inhibitor NVP-LAQ824 sensitizes human nonsmall cell lung cancer to the cytotoxic effects of ionizing radiation. Anticancer Drugs. 2007 Aug;18(7):793-800. PubMed PMID: 17581301.
9: Kato Y, Salumbides BC, Wang XF, Qian DZ, Williams S, Wei Y, Sanni TB, Atadja P, Pili R. Antitumor effect of the histone deacetylase inhibitor LAQ824 in combination with 13-cis-retinoic acid in human malignant melanoma. Mol Cancer Ther. 2007 Jan;6(1):70-81. PubMed PMID: 17237267.
10: Leyton J, Alao JP, Da Costa M, Stavropoulou AV, Latigo JR, Perumal M, Pillai R, He Q, Atadja P, Lam EW, Workman P, Vigushin DM, Aboagye EO. In vivo biological activity of the histone deacetylase inhibitor LAQ824 is detectable with 3′-deoxy-3′-[18F]fluorothymidine positron emission tomography. Cancer Res. 2006 Aug 1;66(15):7621-9. PubMed PMID: 16885362.
SEE MORE AT……….http://drugsynthesisint.blogspot.in/p/nostat-series.html
SEE MORE AT……….http://drugsynthesisint.blogspot.in/p/nostat-series.html
Image may be NSFW.
Clik here to view.
Filed under: PHASE1 Tagged: cancer cell lines, Dacinostat, histone deacetylase inhibitor, LAQ-824, NVP-LAQ824, PHASE 1 Image may be NSFW.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
Clik here to view.
