Originally posted on DRUG REGULATORY AFFAIRS INTERNATIONAL:

The EMA has published together with the FDA a new question & answer (Q&A) paper at the end of 2014. This document answers questions on detailed requirements in connection with the documents concerning regulatory submissions. Among others it contains the answer to the question “What level of detail should be considered for design of experiments (DOEs) in a regulatory submission?”
GMP News
25/02/2015
In our News dated 18 February we reported on a question & answer (Q&A) paper which was published by EMA and FDA together at the end of 2014. This document answers questions on detailed requirements in connection with the documents concerning regulatory submissions. It also answers a question on the topic design of experiments (DOE).

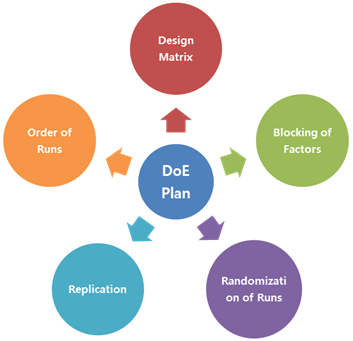
The document answers the question “What level of detail should be considered for design of experiments (DOEs) in a regulatory submission?” as follows:
The level of detail should be commensurate…
View original 165 more words
Filed under: Uncategorized