
CDK Inhibitor, MK 7965, DINACICLIB, SCH 727965
REVIEW…….http://www.mdpi.com/2072-6694/6/4/2224/htm
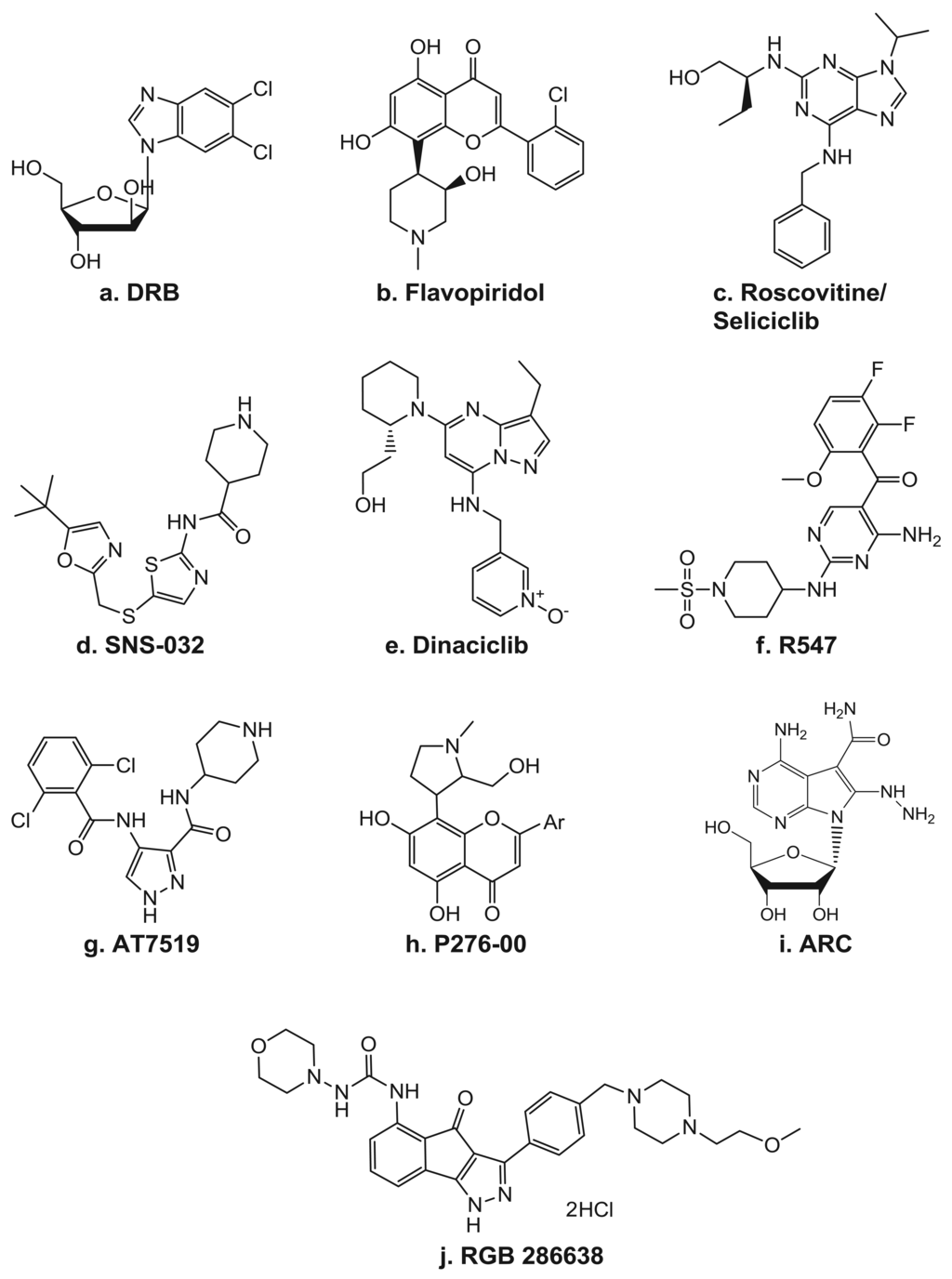
One of the most popular CDK inhibitor in clinical trials in the recent years was dinaciclib (MK-7965, SCH 727965) (Figure 3), the inhibitor of CDK1, CDK2, CDK5, and CDK9. A Phase I trial on the effect of dinaciclib in combination with aprepitant was performed in patients with advanced malignancies [44]. Aprepitant is used for the prevention of chemotherapy-induced nausea and vomiting, is known as an inhibitor and inducer of CYP3A4, which metabolizes dinaciclib.
Coadministration of dinaciclib with aprepitant resulted in no clinically significant effect on the pharmacokinetics and did not alter the safety profile of dinaciclib. The first Phase I clinical trial on dinaciclib as a single agent was performed on patients with advanced malignancies [68]. Forty-eight patients with various solid tumors were treated and 10 of them achieved prolonged stable disease for at least four treatment cycles. Adverse effects were mild, the most common being nausea, anemia, decreased appetite and fatigue.
A phase II multi-center study of dinaciclib for relapsed and/or refractory AML was performed on 20 patients [69]. Temporary decrease in peripheral blood and/or bone marrow blasts was observed in 60% of patients. Four of 13 (31%) patients with circulating blasts had >50% decrease and 6 (46%) >80% decrease in the absolute blast count within 1–8 days of the first dinaciclib dose. Toxicities included diarrhea, fatigue, transaminitis, and manifestations of tumor lysis syndrome, with one patient who deceased of acute renal failure. Another Phase II study was performed of dinaciclib versus erlotinib in patients with non-small cell lung cancer [70].
Unfortunately, it was found that dinaciclib was not successful as monotherapy in non-small cell lung cancer. Most common toxicities included neutropenia, leukopenia, vomiting, and diarrhea. Yet another Phase II study was performed on dinaciclib versus capecitabine in patients with advanced breast cancer [71]. Dinaciclib treatment demonstrated antitumor activity in two of seven patients with ER-positive and ERBB 2-negative metastatic breast cancer, however efficacy was not superior to capecitabine (p = 0.991).
Toxicities included neutropenia, leukopenia, increase in aspartate aminotransferase, and febrile neutropenia. Phase I nonrandomized dose-escalation trial was performed, where patients with relapsed or refractory chronic lymphocytic leukemia were treated with dinaciclib and rituximab [72]. Four out of six patients achieved stable disease, and one patient achieved complete response. Drug-related adverse events were mostly hematological, digestive and metabolic and no dose-limiting toxicities were observed. Dinaciclib was also moved into Phase III development for refractory chronic lymphocytic leukemia [73]. Phase I/II clinical trial Dinaciclib in patients with relapsed multiple myeloma showed promise as single agent [74]. The overall confirmed response rate was 3 of 27 (11%). Adverse effects included leukopenia, thrombocytopenia, gastrointestinal symptoms, alopecia, and fatigue. –
FOR REF See more at: http://www.mdpi.com/2072-6694/6/4/2224/htm#sthash.amBuLwq1.dpuf

Dinaciclib (SCH-727965) is an experimental drug that inhibits cyclin-dependent kinases (CDKs.[1] It is being evaluated in clinical trials for various cancer indications.[2]
Mechanisms of action
- Cyclin-dependent kinase inhibitor dinaciclib interacts with the acetyl-lysine recognition site of bromodomains.[3]
- Dinaciclib (SCH727665) inhibits the unfolded protein response (UPR) through a CDK1 and CDK5-dependent mechanism.[4]
Anti-tumoral action
- In chronic lymphocytic leukemia (CLL)
- In pancreatic cancer
- Dinaciclib inhibits pancreatic cancer growth and progression in murine xenograft models.[7]
- In osteosarcoma
 |
|
| Systematic (IUPAC) name | |
|---|---|
| (S)-3-(((3-Ethyl-5-(2-(2-hydroxyethyl)piperidin-1-yl)pyrazolo[1,5-a]pyrimidin-7-yl)amino)methyl)pyridine 1-oxide | |
| Clinical data | |
| Legal status |
|
| Identifiers | |
| CAS number | 779353-01-4 |
| ATC code | ? |
| PubChem | CID 46926350 |
| ChemSpider | 25027387 |
| ChEMBL | CHEMBL2103840 |
| Synonyms | SCH-727965 |
| Chemical data | |
| Formula | C21H28N6O2 |
Clinical trials
/////////////////////////////////////////////////
http://www.google.com.tr/patents/US8076479
One example of these inhibitors is the compound of Formula II.
The synthesis of the compound of Formula II is described in the ‘878 publication according to Scheme II:
Scheme II:
Step 1—Amidization to Form Substituted Pyrazole
http://www.google.com.tr/patents/US8076479
Step 2—Formation and Dehalogenation of pyrazolo[1,5a]pyrimidine
Step 3—Amination (Two Separate, Sequential Reactions)
As described in the ‘878 publication, Synthetic Scheme II leading to the compound of Formula II has several disadvantages from the standpoint of commercial scale synthesis. In step 1, the starting material (compound “C”) used in the formation of compound “D” is a sticky, viscous oil which is difficult to process (weigh, transfer, and blend). Moreover, step 1, as described in the ‘878 publication, requires isolation and chromatographic purification of compounds C and D prior to carrying out each subsequent derivatization reaction. In addition, as described in the ‘878 publication, the reaction of compound C with malonate diester is carried out using the diester as a solvent. After isolation and purification of the resultant malonate adduct, compound D, ring closure to form diketone compound E is carried out in methanol. In accordance with the procedure described in the ‘878 publication, compound E is isolated and dried, then converted to the corresponding dichloride in N,N-dimethyl aniline by treatment with phosphorous oxychloride (POCl3). The dichloride thus formed was isolated and purified by chromatography prior to the sequential amination reactions. Additionally, the compounds of Formula G and of Formula II require chromatography purification and isolations, as described in the ‘878 publication.
As further described in the ‘878 publication, each of the amination reactions were run separately with isolation and chromatographic purification between amination reactions. Accordingly, the ‘878 publication describes the preparation of the compound of Formula II utilizing a scheme consisting of five separate reaction steps with intervening isolation and purification of the products, each sequential step being carried out in a different solvent system. The overall yield of the compound of Formula II reported for this synthesis, based on starting compound C (Scheme II) is about 20%.
Example 1Preparation of Diketone Compound E (Scheme VI) 3-Ethylpyrazolo[1,5-a]pyrimidine-5,7(4H,6H)-dione
To a 250 ml, three-necked flask equipped with a thermometer, a reflux condenser and mechanical stirrer was charged 3-amino-4-ethylpyrazole oxalate (10 g, 50 mmole), dimethylmalonate (10 ml, 88 mmole), methyl alcohol (80 ml) and sodium methoxide (50 ml, 245 mmole, 25% in methyl alcohol). The batch was heated at reflux for 16 hours then cooled to room temperature. Celite (5 g) and water (60 ml) were added to the batch and agitated for 10 minutes. The batch was filtered to remove the solid residue. The filtrate was pH adjusted to pH˜3 with aqueous HCl (10 ml) to effect precipitation. The precipitate (compound “E”) was filtered and washed with water (40 ml). The wet cake was dried for 18 hours in vacuum oven maintained in the range of oven at 45° C. to 55° C., to give a solid product (84.3%, 7.5 g). C8H9N3O3, Mp: 200-205° C.; NMR in DMSO-d6: 1.05 (t, 3H), 2.23 (q, 2H), 3.26 (bs, 1H), 3.89 (bs, 1H), 7.61 (s, 1H), 11.50(bs, 1H).
Example 2Preparation of Dichloride Compound F (Scheme VI) 5,7-Dichloro-3-Ethylpyrazolo[1,5-a]pyrimidine
Into a 3-neck flask fitted with an inert gas inlet, a reflux condenser and a mechanical stirring apparatus and containing 83 liters of acetonitrile was placed 3-Ethylpyrazolo[1,5-a]pyrimidine-5,7(4H,6H)-dione (E) prepared as described in Step 1 (11.0 kg, 61.5 mole), N,N-dimethylaniline (8.0 L, 63 mole) and POCl3 (7 kg, 430 mole). With stirring the mixture was brought to reflux and maintained under refluxing conditions for 15 hours. The reaction mixture was sampled periodically to monitor the amount of compound “E” present. After the conversion was complete, the solution was cooled to 15° C. Into the cooled reaction mixture was added water which had been cooled to a temperature of less than 20° C. The product is filtered and washed with 4 aliquots of acetonitrile-water (1:3) which had been cooled to a temperature of 20° C. followed by a wash with 10× water. The wet cake is dried in a vacuum oven maintained at 40° C. for at least 15 hours to yield the compound “F” (86.7%); 1H NMR (CDCl3): 1.32(t, 3H), 2.81 (q, 2H), 6.92 (s, 1H), 8.10 (s, 1H)
mp: 90-95° C.
Example 3Preparation of Compound G (Scheme VI) 5-Chloro-3-Ethyl-N-[(1-oxido-pyridinyl)methyl]pyrazolo-[1,5-a]pyrimidine-5.7(4H,6H)-dion-7-amine
Into a 3-liter, three-necked flask equipped with a thermometer, a reflux condenser and mechanical stirrer was charged an aliquot of the dichloride compound “F” prepared in Step 2 (150 g, 0.69 mole), potassium phosphate tribasic monohydrate (338.0 g, 1.47 mole), the dihydrochloride salt of N-oxide-pyridin-3-yl-methylamine, compound F1a (142.5 g, 0.72 mole), water (1500 ml) and acetonitrile (300 ml). The batch was heated at reflux for 6 hours. At the end of the refluxing period the batch was cooled to room temperature over 2 hours and then held at room temperature for 4 hours. The resulting precipitate was filtered and washed with water (600 ml). The wet cake was returned to the flask with water (1500 ml) and acetonitrile (300 ml), and heated to reflux. Reflux was maintained for 6 hours additional. At the end of the second reflux period the reaction mixture was cooled to room temperature over a 2 hour period and left to stand at room temperature for 4 hours. The resulting precipitate was filtered and washed with water (600 ml). The wet cake was dried in an air draft oven at 50° C. for 18 hours to give the first amine adduct “G” material (179 g, 84.9%). mp: 187-189C; NMR in CDCl3, 1.26(t, 3H), 2.73(q, 2H), 4.60(d, 2H), 5.87(s, 1H), 6.83(bs, 1H), 7.33(t, 1H), 7.70(d, 1H), 7.84(s, 1H), 8.58(d, 1H), 8.64(d, 1H).
Example 4
Preparation of the Compound of Formula II (Scheme VI) 1-[3-Ethyl-7-[(1-oxido-3-pyridinyl)methyl]amino]pyrazolo[1,5-a]pyrimidin-5-yl]-2(s)-piperidinemethanol
Into a three-neck flask fitted with a mechanical stirrer and a reflux condenser were placed the first amine adduct prepared in Step 3, compound “G”, (7 kg, 23 mole), amino-alcohol compound G1a (5.6 kg, 43.3 mole), sodium carbonate (3.5 kg, 33.0 mole), 110 ml of water and 1-methyl-2-pyrrolidinone (NMP) (11 L). The reaction mixture was heated to 150° C. for 4 days. After chromatography indicated that the reaction was complete (90-95% substrate consumed), the reaction mixture was cooled to room temperature and quenched by adding water. The mixture was then extracted with ethyl acetate. The batch was dried by distillation of the water azeotrope under atmospheric pressure and concentrated to about 28 L volume. THF was added and the solution was heated to reflux until all the solids dissolve. Ethyl acetate and trietylamine are added to the hot solution. The batch was cooled to ambient and then agitated with the temperature maintained in the range of from 20° C. to 25° C. for 12 hours. The solids were collected by filtration, washed first with ethyl acetate then water, and dried in the filter under vacuum for 24 hours with the temperature maintained at from 40° C. to 50° C., yielding 4.9 kg, 51.3% of the compound of Formula II.
DSC, 168.6° C.; Specific Rotation (10 mg/ml in MeOH, 20° C.), −117.8 °;
1HNMR (400 MHz, DMSO): 8.31 ppm (1H, s), 8.11-8.13 ppm (1H, td, J=5.7 Hz, J=1.4 Hz), 7.97 ppm (1H, t, J=6.7 Hz), 7.68 ppm (1H, s), 7.41 ppm (1H, s), 7.37-7.43 ppm (1H, dd), 5.55 ppm (1H, s), 4.85 ppm (1H, t, J=5.4 Hz), 4.49-4.59 ppm (3H, m), 4.24-4.28 ppm (1H, broad), 3.27-3.46 ppm (2H, m), 2.76-2.83 ppm (1H, t, J=13.0 Hz), 2.45-2.50 ppm (2H, q, J=7.5 Hz), 1.72-1.79 (1H, m), 1.54-1.68 ppm (6H, m), 1.30-1.34 ppm (1H, m), 1.16 ppm (3H, t, J=7.5 Hz)
References
- Parry, D; Guzi, T; Shanahan, F; Davis, N; Prabhavalkar, D; Wiswell, D; Seghezzi, W; Paruch, K; Dwyer, M. P.; Doll, R; Nomeir, A; Windsor, W; Fischmann, T; Wang, Y; Oft, M; Chen, T; Kirschmeier, P; Lees, E. M. (2010). “Dinaciclib (SCH 727965), a novel and potent cyclin-dependent kinase inhibitor”. Molecular Cancer Therapeutics 9 (8): 2344–53. doi:10.1158/1535-7163.MCT-10-0324. PMID 20663931.
- Jump up^ Bose P, Simmons GL, Grant S (2013). “Cyclin-dependent kinase inhibitor therapy for hematologic malignancies”. Expert Opin Investig Drugs 22 (6): 723–38.doi:10.1517/13543784.2013.789859. PMC 4039040. PMID 23647051.
- Martin, M. P.; Olesen, S. H.; Georg, G. I.; Schönbrunn, E (2013). “Cyclin-dependent kinase inhibitor dinaciclib interacts with the acetyl-lysine recognition site of bromodomains”. ACS Chemical Biology 8 (11): 2360–5. doi:10.1021/cb4003283. PMC 3846258. PMID 24007471.
- Nguyen, T. K.; Grant, S (2013). “Dinaciclib (SCH727665) inhibits the unfolded protein response (UPR) through a CDK1 and CDK5-dependent mechanism”. Molecular Cancer Therapeutics 13(3): 662–74. doi:10.1158/1535-7163.MCT-13-0714. PMID 24362465.
- Jump up^ Desai, B. M.; Villanueva, J; Nguyen, T. T.; Lioni, M; Xiao, M; Kong, J; Krepler, C; Vultur, A; Flaherty, K. T.; Nathanson, K. L.; Smalley, K. S.; Herlyn, M (2013). “The anti-melanoma activity of dinaciclib, a cyclin-dependent kinase inhibitor, is dependent on p53 signaling”. PLoS ONE 8 (3): e59588. doi:10.1371/journal.pone.0059588. PMC 3601112. PMID 23527225.
- Jump up^ Johnson, A. J.; Yeh, Y. Y.; Smith, L. L.; Wagner, A. J.; Hessler, J; Gupta, S; Flynn, J; Jones, J; Zhang, X; Bannerji, R; Grever, M. R.; Byrd, J. C. (2012). “The novel cyclin-dependent kinase inhibitor dinaciclib (SCH727965) promotes apoptosis and abrogates microenvironmental cytokine protection in chronic lymphocytic leukemia cells”. Leukemia 26 (12): 2554–7.doi:10.1038/leu.2012.144. PMC 3645353. PMID 22791353.
- Jump up^ Feldmann, G; Mishra, A; Bisht, S; Karikari, C; Garrido-Laguna, I; Rasheed, Z; Ottenhof, N. A.; Dadon, T; Alvarez, H; Fendrich, V; Rajeshkumar, N. V.; Matsui, W; Brossart, P; Hidalgo, M; Bannerji, R; Maitra, A; Nelkin, B. D. (2011). “Cyclin-dependent kinase inhibitor Dinaciclib (SCH727965) inhibits pancreatic cancer growth and progression in murine xenograft models”.Cancer biology & therapy 12 (7): 598–609. PMC 3218385. PMID 21768779.
- Jump up^ Fu, W; Ma, L; Chu, B; Wang, X; Bui, M. M.; Gemmer, J; Altiok, S; Pledger, W. J. (2011). “The cyclin-dependent kinase inhibitor SCH 727965 (dinacliclib) induces the apoptosis of osteosarcoma cells”. Molecular Cancer Therapeutics 10 (6): 1018–27. doi:10.1158/1535-7163.MCT-11-0167. PMID 21490307.
- Jump up^ Fu, W; Sharma, S. S.; Ma, L; Chu, B; Bui, M. M.; Reed, D; Pledger, W. J. (2013). “Apoptosis of osteosarcoma cultures by the combination of the cyclin-dependent kinase inhibitor SCH727965 and a heat shock protein 90 inhibitor”. Cell Death and Disease 4 (3): e566. doi:10.1038/cddis.2013.101. PMC 3613821. PMID 23538447.
- Jump up^ Nemunaitis, J. J.; Small, K. A.; Kirschmeier, P; Zhang, D; Zhu, Y; Jou, Y. M.; Statkevich, P; Yao, S. L.; Bannerji, R (2013). “A first-in-human, phase 1, dose-escalation study of dinaciclib, a novel cyclin-dependent kinase inhibitor, administered weekly in subjects with advanced malignancies”. Journal of Translational Medicine 11 (1): 259. doi:10.1186/1479-5876-11-259.PMC 3853718. PMID 24131779.
- Jump up^ Mita, M; Joy, A. A.; Mita, A; Sankhala, K; Jou, Y. M.; Zhang, D; Statkevich, P; Zhu, Y; Yao, S. L.; Small, K; Bannerji, R; Shapiro, C. L. (2013). “Randomized Phase II Trial of the Cyclin-Dependent Kinase Inhibitor Dinaciclib (MK-7965) Versus Capecitabine in Patients with Advanced Breast Cancer”. Clinical Breast Cancer 14 (3): 169–76. doi:10.1016/j.clbc.2013.10.016.PMID 24393852.
- Jump up^ Stephenson, J. J.; Nemunaitis, J; Joy, A. A.; Martin, J. C.; Jou, Y. M.; Zhang, D; Statkevich, P; Yao, S. L.; Zhu, Y; Zhou, H; Small, K; Bannerji, R; Edelman, M. J. (2014). “Randomized phase 2 study of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus erlotinib in patients with non-small cell lung cancer”. Lung Cancer 83 (2): 219–23.doi:10.1016/j.lungcan.2013.11.020. PMID 24388167.
External links
- dinaciclib at the US National Library of Medicine Medical Subject Headings (MeSH)

Patent Submitted Granted
Process and intermediates for the synthesis of (3-alkyl-5-piperidin-1-yl-3,3a-dihydro-pyrazolo[1,5-a]pyrimidin-7-yl)-amino derivatives and intermediates [US8076479]2008-03-06 GRANT2011-12-13
Process for resolving chiral piperidine alcohol and process for synthesis of pyrazolo[1,5-a] pyrimidine derivatives using same [US7786306]2008-02-28 GRANT2010-08-31
Sequential Administration of Chemotherapeutic Agents for Treatment of Cancer [US2011129456]2011-06-02
TARGETING CDK4 AND CDK6 IN CANCER THERAPY [US2011009353]2011-01-13
Pyrazolopyrimidines as cyclin dependent kinase inhibitors [US2007225270]2007-09-27
PYRAZOLO[1,5-a]PYRIMIDINES [US2007275963]2007-11-29
Novel pyrazolopyrimidines as cyclin dependent kinase inhibitors [US2007281951]2007-12-06
Novel pyrazolopyrimidines as cyclin dependent kinase inhibitors [US2008050384]2008-02-28
Novel pyrazolopyrimidines as cyclin dependent kinase inhibitors [US2007054925]2007-03-08
Filed under: Anthony crasto, Phase3 drugs Tagged: CDK Inhibitor, DINACICLIB, MK 7965, PHASE 3, SCH 727965







