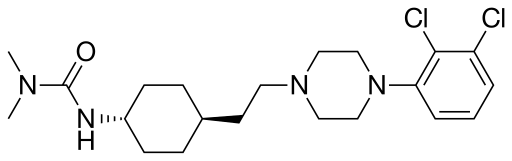
CARIPRAZINE
CAS 839712-12-8 (free base)
CAS 1083076-69-0…HYDROCLORIDE SALT
trans-N-[4-[2-[4-(2,3-Dichlorophenyl)piperazin-1-yl]ethyl]cyclohexyl]-N’,N’-dimethylurea
Trans-1-{4-[2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-ethyl]-cyclohexyl}-3,3- dimethyl-urea
trans-4-{2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-ethyl}-N,N-dimethylcarbamoyl-cyclohexylamine
trans-1{4-[2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-ethyl]-cyclohexyl}-3,3-dimethyl-urea,
3-(trans-4-(2-(4-(2,3-Dichlorophenyl)piperazin-1-yl)ethyl)cyclohexyl)-1,1-dimethylurea
IN PHASE 3 FOR MAJOR DEPRESSION
Cariprazine (RGH-188) is an antipsychotic drug under development by Gedeon Richter. It acts as a D2 and D3 receptor partial agonist, with high selectivity towards the D3 receptor.[1] Positive Phase III study results were published for schizophrenia and maniaearly 2012, while Phase II studies in bipolar disorder I, and for bipolar depression are in progress.[2] Action on the dopaminergic systems makes it also potentially useful as an add-on therapy in major depressive disorder [3]
Forest Laboratories obtained a license on development (from the Richter – Hungary) and exclusive commercial rights in the US in 2004.

 NEWS………….DUBLIN and BUDAPEST, Hungary, Jan. 6, 2015 /PRNewswire/ — Actavis plcand Gedeon Richter Plc. today announced that the U.S. Food and Drug Administration (FDA) has acknowledged receipt of Actavis’ New Drug Application (NDA) resubmission for its atypical antipsychotic cariprazine, a potent dopamine D3/D2 receptor partial agonist with preferential binding to D3 receptors. The Prescription Drug User Fee Act (PDUFA) date is expected to be in the second quarter of 2015…….
NEWS………….DUBLIN and BUDAPEST, Hungary, Jan. 6, 2015 /PRNewswire/ — Actavis plcand Gedeon Richter Plc. today announced that the U.S. Food and Drug Administration (FDA) has acknowledged receipt of Actavis’ New Drug Application (NDA) resubmission for its atypical antipsychotic cariprazine, a potent dopamine D3/D2 receptor partial agonist with preferential binding to D3 receptors. The Prescription Drug User Fee Act (PDUFA) date is expected to be in the second quarter of 2015…….

Production building of the company in Budapest
Medical uses
Cariprazine is currently in clinical trials for schizophrenia and bipolar disorder. It has also been investigated as a potential adjunct in treatment-resistant major depressive disorder.[4]

Illustrated Pill Packaging
Side effects
The most prevalent side effects for cariprazine include akathisia, insomnia, and weight gain. Cariprazine does not appear to impact metabolic variables or prolactin levles, and unlike many other antipsychotics, does not increase the electrocardiogram (ECG) QT interval. In short term clinical trials extrapyramidal effects, sedation, akathisia, nausea, dizziness, vomiting, anxiety, and constipation were observed. One review characterized the frequency of these events as “not greatly different from that seen in patient treated with placebo”[5] but a second called the incidence of movement-related disorders “rather high”[6][7] .
Pharmacodynamics
Cariprazine acts as an antipsychotic that is effective against the positive and negative symptoms of schizophrenia.[8] Unlike many antipsychotics that are D2 and 5-HT2A receptor antagonists, cariprazine is a D2 and D3 partial agonist. It also has a higher affinity for D3 receptors. The D2 and D3 receptors are important targets for the treatment of schizophrenia, because the overstimulation of dopamine receptors has been implicated as a possible cause of schizophrenia.[9] Cariprazine acts to inhibit overstimulated dopamine receptors (acting as an antagonist) and stimulate the same receptors when the endogenous dopamine levels are low. Cariprazine’s high selectivity towards D3 receptors could prove to reduce side effects associated with the other antipsychotic drugs, because D3receptors are mainly located in the ventral striatum and would not incur the same motor side effects (extrapyramidal symptoms) as drugs that act on dorsal striatum dopamine receptors.[8] Cariprazine also acts on 5-HT1A receptors, though the affinity is considerably lower than the affinity to dopamine receptors (seen in monkey and rat brain studies).[8][10] In the same studies, cariprazine has been noted to produce pro-cognitive effects, the mechanisms of which are currently under investigation. An example of pro-cognitive effects occurred in pre-clinical trials with rats: rats with cariprazine performed better in a scopolamine-induced learning impairment paradigm in a water labyrinth test. This may be due to the selective antagonist nature of D3 receptors, though further studies need to be conducted.[8] This result could be very useful for schizophrenia, as one of the symptoms includes cognitive deficits.
Cariprazine has partial agonist as well as antagonist properties depending on the endogenous dopamine levels. When endogenous dopamine levels are high (as is hypothesized in schizophrenic patients), cariprazine acts as an antagonist by blocking dopamine receptors. When endogenous dopamine levels are low, cariprazine acts more as an agonist, increasing dopamine receptor activity.[11] In monkey studies, the administration of increasing does of cariprazine resulted in a dose-dependent and saturable reduction of specific binding. At the highest dose (300 μg/kg), the D2/D3 receptors were 94 % occupied, while at the lowest dose (1 μg/kg), receptors were 5 % occupied.[10]
| Receptor | Ki (nM)[4] | Pharmacodynamic action[4] |
|---|---|---|
| 5-HT1A | 3 | Partial agonism |
| 5-HT2A | 19 | Inverse agonism/antagonism |
| 5-HT2B | 0.58 | Inverse agonism/Antagonism |
| 5-HT2C | 134 | Inverse agonism/Antagonism |
| 5-HT7 | 111 | Antagonism |
| D2S | 0.69 | Partial agonism |
| D2L | 0.49 | Partial agonism |
| D3 | 0.085 | Partial agonism |
| H1 | 23 | Inverse agonism/antagonism |
Pharmacokinetics
Cariprazine has high oral bioavailability and can cross the blood brain barrier easily in humans because it is lipophilic.[2] In rats, the oral bioavailability was 52 % (with a dose of 1 mg/kg).[7]
………………………
PATENT
http://www.google.com/patents/EP1663996A1?cl=en
Trans-1-{4-[2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-ethyl]-cyclohexyl}-3,3- dimethyl-urea (compound 1 )
Example 1 1-(2,3-dichlorophenyl)-[1,4]diazepine (starting material)
2.25 g (10 mmol) 1-bromo-2,3-dichloro-benzene was dissolved in dry toluene (50 ml), 2.3 (11 mmol) of [1 ,4]diazepine-1 -carboxylic acid tert-butylester was added followed by 0.2 g BINAP (2,2-bis(diphenylρhosphino)-1 ,1′-binaphtyl), 85 mg tris(dibenzylideneacetone)dipalladium(0) and 1.2 g (12mmol) sodium-tert-butoxyde. The reaction mixture was refluxed for eight hours and filtered. The organic layer was washed with water, dried and evaporated in vacuo. The residue was purified by chromatography and deprotected at 10 °C using 20 ml ethylacetate saturated with gaseous hydrochloric acid, the precipitate was filtered giving 2.1 g (yield: 75 %) hydrochloride salt of the title compound, melting at 182-3 °C. Example 2 Trans-N-{4-[2-[4-(2,3-dichloro-phenyl)-hexahydro-[1 ,4]diazepin-1-yl]-ethyl]- cyclohexyl}-carbamic acid tert-butylester (intermediate) 0.7 g (2.5 mmol) of 1 -(2,3-dichlorophenyl)-[1 ,4]diazepine hydrochloride and
0.6 g (2.5 mmol) of frat?s-2-{1 -[4-(N-tert-butyloxycarbonyl)amino]cyclohexyl}- acetaldehyde were dissolved in dichloroethane (35 ml), 0.35 ml (2.5 mmol) triethylamine was added, then 0.79 g (3.7 mmol) sodium triacetoxyborohydride was added portionswise and the reaction mixture was stirred for 20 hours at ambient temperature, then 20 % potassium carbonate solution in water (20 ml) was added. The organic layer was separated, dried and evaporated to dryness in vacuo. The precipitate was recrystallized from acetonitrile to give the title compound 1 .0 g (yield: 85.8 %), m.p.: 95-8 °C. Example 3
Trans-4-[2-[4-(2,3-dichloro-phenyl)-hexahydro-[1 ,4]diazepin-1-yl]-ethyl]- cyclohexylamine (intermediate)
0.93 g (2.1 mmol) frarjs-N-{4-[2-[4-(2,3-dichloro-phenyl)-hexahydro- [1 ,4]diazepin-1 -yl]-ethyl]-cyclohexyl}-carbamic acid tert-butylester was deprotected at
10 °C using 15 ml ethylacetate saturated with gaseous hydrochloric acid, after 4 hours the precipitate was filtered giving 0.91 g (yield: 98 %) dihydrochloride salt of the title compound, melting at 260-6 °C. Method A
Trans-1-{4-[2-[4-(2,3-dichlorophenyl)-piperazin-1-yi]-ethyl]-cyclohexyl}-3,3- dimethyl-urea (compound 1 ) 1 .39g (3 mmol) trans-4-{2-[4-(2,3-dichlorophenyl)-ρiperazin-1 -yl]-ethyl}- cyclohexyl-amine trihydrochloride was suspended in dichloromethane (100 ml), triethylamine (2.1 ml, 15 mmol) was added followed by 0.30 ml (3.3 mmol) N,N- dimethylcarbamoylchloride. The reaction mixture was stirred for 48 hours at room temperature, filtered. The filtrate was washed with water (2 x 20 ml), dried and evaporated in vacuo. Recrystallizing from methanol gave the title compound (0.83 g, 65 %), melting at 212-4 °C.
Method B
7rans-1-{4-[2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-ethyl]-cyclohexyl}-3-ethyl- urea (compound 2) 0.56g (1.2 mmol) trans-4-{2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-ethyl}- cyclohexyl-amine was dissolved in dry dichloromethane (20 ml), ethylisocyanate (0.1 ml, 1.3 mmol) was added and the reaction mixture was stirred at room temperature for 4 hours. The solvent was removed in vacuo. The residue was stirred with water, the precipitate was filtered, giving the title compound (0.33 g, 65 %). Melting point:
235-8 °C.
Method C rrans-1-{4-[2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-ethyl]-cyclohexyl}-3,3- dimethyl-urea (compound 1 )
0.56g (1.2 mmol) trans-4-{2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-ethyl}- cyclohexyl-amine trihydrochloride was suspended in dry dichloromethane (50 ml), triethylamine 0.77 ml, 6 mmol) was added and 0.13g (0.44 mmol) triphosgene dissolved in dichloromethane was dropped in. After one hour stirring at room temperature dimetilamine hydrochloride (0.49 g, 6 mmol) followed by triethylamine (0.84 ml, 6 mmol) was added and the stirring was continued for 20 hours. The mixture was filtered, the filtrate washed with water, dried and evaporated in vacuo. Recrystallizing the product from methanol gave the title compound (0.27 g, 52 %). Melting point: 212-4 °C.

……………………
PATENT
http://www.google.com/patents/US20090023750
U.S. Patent Publication No. 2006/0229297 discloses (thio)-carbamoyl-cyclohexane derivatives that are D3 and D2 dopamine receptor subtype preferring ligands, having the formula (I):
(I)
wherein R1, R2, X, and n are as defined therein. One particular compound disclosed therein is trans-1{4-[2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-ethyl]-cyclohexyl}-3,3-dimethyl-urea, which is also known as trans-4-{2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-ethyl}-N,N-dimethylcarbamoyl-cyclohexylamine, the structural formula for which is shown below:
Compounds of formula (I) act as a dopamine receptor antagonists, particularly D3/D2 receptor antagonists, and are useful in the treatment and prevention of pathological conditions which require modulation of dopamine receptors.
In some cases, an appropriate salt of an active may improve certain properties suitable for pharmaceutical compounds (i.e., stability, handling properties, ease of large scale synthesis, etc.). However, selection of a suitable salt for a particular active agent is not always straightforward, since the properties of salts of different compounds formed with the same salt forming agent may differ greatly. Moreover, formation of particular salts of a compound possessing more than one basic centre may be difficult to achieve in high yield due to formation of multiple products.
…………………..
see
WO 2011073705
http://www.google.com/patents/WO2011073705A1?cl=en
We have surprisingly found that by reacting trans 4-{2-[4-(2,3-dichlorophenyl)- piperazine-l-yl]-ethyl}-cyclohexylamine of formula (III)
with a carbonic acid derivative of general formula (VI)R-O-CO-Z (VI)
then reacting the compound of general formula (IV) obtained
with an amine derivative of general formula (V)
get the compounds of general formula (I)
EXAMPLES
The invention is illustrated by the following non-limiting examples.
Example 1
Trans N-(4-{2-[4-(2,3-dichlorophenyl)-piperazine-l-yl]-ethyl}-cyclohexyl)-carbamic acid methylester 6.45 g (0.015 mol) of dihydrochloride of compound of formula (III) was added to a mixture of 125 ml dichloromethane and 12.25 ml triethylamine and the thick suspension obtained was stirred at a temperature between 20-25°C for one hour. The so obtained suspension was added to a solution of 2.3 ml (0.03 mol) methyl chloroformate in 25 ml of dichloromethane at a temperature between 5-10°C. The reaction mixture obtained was stirred at a temperature between 20-25°C for 3 hours then extracted with 3×150 ml (150 g) of distilled water. The organic phase was evaporated in vacuum and the residue was recrystallized from methanol. In this manner 4.5 g of the title product was obtained.
Yield: 72 %.
Melting point: 143-147 °C
Example 2
Trans N-(4-{2-[4-(2,3-dichlorophenyl)-piperazine-l-yl]-ethyl}-cyclohexyl)-carbamic acid isopropylester
6.45 g (0.015 mol) of dihydrochloride of compound of formula (III) was added to a mixture of 125 ml dichloromethane and 12.25 ml of triethylamine and the thick suspension obtained was stirred at a temperature between 20-25°C-on for one hour. The suspension was added to a solution of 3.7 g (0.03 mol) of isopropyl chloroformate in 30 ml of toluene at a temperature between 5-10°C. The reaction mixture was stirred at a temperature between 20-25°C for 3 hours and then extracted with 3×150 ml (150 g) of distilled water. The organic phase was evaporated in vacuum and the residue obtained was recrystallized from isopropanole.
In this manner 4,4 g of title compound was obtained. Yield: 67 %.
Melting point: 128-131°C
Example 3
Trans 4-{2-[4-(2,3-dichlorophenyl)-piperazine-l-yl]-ethyl}-N,N-dimethylcarbamoyl- cyclohexylamine
6.45 g (0.015 mol) of dihydrochloride of compound of formula (III) was added to a mixture of 125 ml of dichloromethane and 12.25 ml of triethylamine and the thick suspension obtained was stirred at a temperature between 20-25°C for one hour. The suspension was added to a solution of 4.9 g of bis(trichloromethyl)carbonate in 50 ml of dichloromethane at a temperature between -5-(-10)°C for one hour. The reaction mixture obtained was added to a solution of 13 g dimethylamine in 100 ml isopropyl alcohol (IP A) (40 ml, 0.12 mol) cooled at a temperature between 0-(-10)°C during which the temperature of the reaction mixture was kept under 0°C. After stirring at a temperature between 0-(-5)°C for 30 minutes to the reaction mixture 100 ml of distilled water was added under stirring. Then the pH of the aqueous phase was adjusted to 7-8 by adding concentrated hydrochloric acid and volume of the reaction mixture was concentrated to 130 ml under vacuum. To the reaction mixture obtained additional 70 ml of distilled water was added and the mixture was concentrated to 170 ml under vacuum. The suspension was stirred at 20-25°C for one hour and the product obtained was isolated by filtration.
In this manner 6.6 g of title compound was obtained.
Yield: 95 %
Melting point: 208-211 °C Example 4
Trans 4-{2-[4-(2,3-dichlorophenyl)-piperazine-l-yl]-ethyI}-N,N-dimethylcarbamoyl- cyclohexylamine 4.4 g (0.011 mol) of trans N-(4-{2-[4-(2,3-dichlorophenyl)-piperazine-l-yl]-ethyl}- cyclohexyl)-carbamic acid methylester was dissolved in 120 ml of dichloromethane. The solution obtained was added to a solution of 13 g dimethylamine in 100 ml isopropyl alcohol (IP A) (100 ml, 0.3 mol) cooled at a temperature between 0-(-10)°C during which the temperature of the reaction mixture was kept under 0°C. After stirring at a temperature between 0-(-5)°C for 30 minutes to the reaction mixture 100 ml of distilled water was added under stirring. Then the pH of the aqueous phase was adjusted to 7-8 by adding concentrated hydrochloric acid and volume of the reaction mixture was concentrated to 100 ml under vacuum. To the reaction mixture obtained additional 70 ml of distilled water was added and the mixture was concentrated to 120 ml under vacuum. The suspension was stirred at 20-25°C for one hour and the product obtained was isolated by filtration.
In this manner 4.3 g of title compound was obtained.
Yield: 95 %
Melting point: 208-211 °C
Example 5
Trans 4-{2-[4-(2,3-dichlorophenyl)-piperazine-l-yl]-ethyl}-N,N-dimethylcarbamoyl- cyclohexylamine hydrochloride 6.45 g (0.015 mol) dihydrochloride of formula (III) was added to a mixture of 125 ml of dichloromethane and 12.25 ml of triethylamine and the thick suspension obtained was stirred at a temperature between 20-25°C for one hour. The suspension was added to the solution of 4.9 g of bis(trichloromethyl)carbonate in 50 ml of dichloromethane at a temperature between -5-(-10)°C for one hour. The reaction mixture obtained was added to a solution of 13 g dimethylamine in 100 ml isopropyl alcohol (IP A) (40 ml, 0.12 mol) cooled at a temperature between 0-(-10)°C during which the temperature of the reaction mixture was kept under 0°C. After stirring at a temperature between 0-(-5)°C for 30 minutes 100 ml of distilled water was added to the reaction mixture under stirring. Then the pH of the aqueous phase is adjusted to 2-3 by adding concentrated hydrochloric acid and the reaction mixture was concentrated to 130 ml, additional 70 ml of distilled water was added and the mixture was concentrated to 170 ml. The suspension was stirred at 20-25°C for one hour and the product obtained was isolated by filtration.
In this manner 6.7 g of title compound was obtained.
Yield: 96 %
Melting point: 221-224 °C
Example 6
Trans 4-{2-[4-(2,3-dichlorophenyl)-piperazine-l-yl]-ethyl}-N,N-dimethylcarbamoil- cyclohexylamine hydrochloride 6.72 g (0.015 mol) dihydrochloride monohydrate of compound of formula (III) was added to a mixture of 125 ml of dichloromethane and 12.25 ml of triethylamine and the thick suspension obtained was stirred at a temperature between 20-25 °C for one hour. The suspension was added to the solution of 4.9 g of bis(trichloromethyl)carbonate in 50 ml of dichloromethane at a temperature between -5-(-10)°C for one hour. The reaction mixture obtained was added to a solution of 13 g dimethylamine in 100 ml isopropyl alcohol (IP A) (40 ml, 0,12 mol) cooled at a temperature between 0-(-10)°C during which the temperature of the reaction mixture was kept under 0°C. After stirring at a temperature between 0-(-5)°C for 30 minutes to the reaction mixture 100 ml of distilled water was added and the pH of the aqueous phase was adjusted to 2-3 by adding concentrated hydrochloric acid. The reaction mixture was concentrated to 130 ml under vacuum then additional 70 ml of water was added and the mixture was concentrated to 170 ml. The suspension was stirred at a temperature between 20-25°C for one hour and the product obtained was isolated by filtration.
In this manner 6.7 g of title compound was obtained.
Yield: 96 %.
Melting point: 221-224 °C
………………………………………
SEE
http://www.google.com/patents/WO2014031162A1?cl=en
……………………………….
PAPER
Bioorganic & Medicinal Chemistry Letters
Volume 22, Issue 10, (15 May 2012)
-
Discovery of cariprazine (RGH-188): A novel antipsychotic acting on dopamine D3/D2 receptors
- Pages 3437-3440
-
Cariprazine, a potential atypical antipsychotic agent has been identified during the optimization of novel series of 4-aryl-piperazine derivatives. The recently available top line results from pivotal clinical trials demonstrated the safety and efficacy of cariprazine in bipolar mania and schizophrenia indications.
![image image]()
………………………………………….
Journal of Medicinal Chemistry, 2013 , vol. 56, 22 pg. 9199 – 9221
http://pubs.acs.org/doi/abs/10.1021/jm401318w

Biased agonism offers an opportunity for the medicinal chemist to discover pathway-selective ligands for GPCRs. A number of studies have suggested that biased agonism at the dopamine D2 receptor (D2R) may be advantageous for the treatment of neuropsychiatric disorders, including schizophrenia. As such, it is of great importance to gain insight into the SAR of biased agonism at this receptor. We have generated SAR based on a novel D2R partial agonist, tert-butyl (trans-4-(2-(3,4-dihydroisoquinolin-2(1H)-yl)ethyl)cyclohexyl)carbamate (4). This ligand shares structural similarity to cariprazine (2), a drug awaiting FDA approval for the treatment of schizophrenia, yet displays a distinct bias toward two different signaling end points. We synthesized a number of derivatives of 4 with subtle structural modifications, including incorporation of cariprazine fragments. By combining pharmacological profiling with analytical methodology to identify and to quantify bias, we have demonstrated that efficacy and biased agonism can be finely tuned by minor structural modifications to the head group containing the tertiary amine, a tail group that extends away from this moiety, and the orientation and length of a spacer region between these two moieties.
3-(trans-4-(2-(4-(2,3-Dichlorophenyl)piperazin-1-yl)ethyl)cyclohexyl)-1,1-dimethylurea (2).(ref…………Ágai-Csongor, É.; Domány, G.; Nógrádi, K.; Galambos, J.; Vágó, I.; Keserű, G. M.; Greiner, I.; Laszlovszky, I.; Gere, A.; Schmidt, É.; Kiss, B.; Vastag, M.; Tihanyi, K.; Sághy,K.; Laszy, J.; Gyertyán, I.; Zájer-Balázs, M.; Gémesi, L.; Kapás, M.; Szombathelyi,Z.Discovery of cariprazine (RGH-188): A novel antipsychotic acting on dopamine D3/D2receptors Bioorg. Med. Chem. Lett. 2012, 22, 3437– 3440)



0.8 g (1.6 mmol) trans-1-{4-[2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-ethyl]-cyclohexyl}-3,3-dimethyl-urea was dissolved in dichloromethane (60 ml). A solution of 0.54 g (2.4 mmol) 3-chloro-perbenzoic acid in dichloromethane (10 ml) was dropped in and the reaction mixture stirred for 24 hours at room temperature. The reaction was monitored by TLC. The solution was washed twice with saturated NaHCO3 solution, the organic layer dried and evaporated in vacuo. Flash chromatography gave 0.45 g (63.3%) of the title compound melting at 175-8° C.
Example 2Trans-1-{4-[2-[4-(2,3-dichloro-4-hydroxy-phenyl)-piperazin-1-yl]-ethyl]-cyclohexyl}-3,3-dimethyl-urea (compound C)
0.92 g (2 mmol) trans-4-{2-[4-(2,3-dichloro-4-methoxy-phenyl)-piperazin-1-yl]-ethyl}-cyclohexyl-amine dihydrochloride was suspended in dichloromethane (60 ml), triethylamine (1.26 ml, 9 mmol) was added followed by 0.21 ml (2.3 mmol) N,N-dimethylcarbamoylchloride. The reaction mixture was stirred for 48 hours at room temperature. The solution was washed with water (2×10 ml), dried and evaporated in vacuo. Purification with flash chromatography gave 0.66 g trans-1-{4-[2-[4-(2,3-dichloro-4-methoxy-phenyl)-piperazin-1-yl]-ethyl]-cyclohexyl}-3,3-dimethyl-urea, melting at 196-8° C. This product was dissolved in dichloromethane (60 ml), then 6.4 ml (6.4 mmol) borontribromid solution (1M in CH2Cl2) was dropped in at 5° C. and the mixture stirred at room temperature for 24 hours. The reaction was monitored by TLC. 4 ml methanol was added, followed by 25 ml saturated NaHCO3 solution. After separation the organic layer was dried and evaporated in vacuo. Purification with flash chromatography gave 0.4 g of the title compound, melting at 278-80° C.
Example 3Trans-1-{4-[2-[4-(2,3-dichloro-4-hydroxy-phenyl)-piperazin-1-yl]-ethyl]-cyclohexyl}-3-methyl-urea (compound B)
1.38 g (3 mmol) trans-4-{2-[4-(2,3-dichloro-4-methoxy-phenyl)-piperazin-1-yl]-ethyl}-cyclohexyl-amine dihydrochloride was suspended in dry dichloromethane (100 ml), triethylamine (1.72 ml, 12.4 mmol) was added and 0.34 g (1.14 mmol) triphosgene dissolved in dichloromethane was dropped in. After one hour stirring at room temperature methylamine (33% solution in ethanol) was added and the stirring was continued for 20 hours. The mixture was evaporated. 20 ml water was added, the precipitate filtered, washed with water, dried. Recrystallizing the product from methanol gave trans-1-{4-[2-[4-(2,3-dichloro-4-methoxy-phenyl)-piperazin-1-yl]-ethyl]-cyclohexyl}-3-methyl-urea (0.86 g, 65%) melting above 250° C. This product was dissolved in dichloromethane (60 ml), then 10 ml (10 mmol) borontribromid solution (1M in CH2Cl2) was dropped in at 5° C. and the mixture stirred at room temperature for 24 hours. The reaction was monitored by TLC. 4 ml methanol was added and the mixture evaporated. 35 ml saturated NaHCO3 solution was added. The precipitate was filtered, washed with water and dried, recrystallized from methanol giving 0.34 g of title compound, melting at 237-41° C.
Example 4Trans-1-{4-[2-[4-(2,3-dichloro-4-hydroxy-phenyl)-piperazin-1-yl]-ethyl]-cyclohexyl}-urea (compound A)
1.38 g (3 mmol) trans-4-{2-[4-(2,3-dichloro-4-methoxy-phenyl)-piperazin-1-yl]-ethyl}-cyclohexyl-amine dihydrochloride was suspended in dry dichloromethane (100 ml), triethylamine 1.72 ml, 12.4 mmol) was added and 0.34 g (1.14 mmol) triphosgene dissolved in dichloromethane was dropped in. After one hour stirring at room temperature ammonia (20% solution in methanol) was added and the stirring was continued for 20 hours. The mixture was evaporated. 20 ml water was added, the precipitate filtered, washed with water, dried. Recrystallizing the product from methanol gave 0.86 g trans-1-{4-[2-[4-(2,3-dichloro-4-methoxy-phenyl)-piperazin-1-yl]-ethyl]-cyclohexyl}-urea melting above 250° C. This product was dissolved in dichloromethane (60 ml), then 10 ml (10 mmol) borontribromid solution (1M in CH2Cl2) was dropped in at 5° C. and the mixture stirred at room temperature for 24 hours. The reaction was monitored by TLC. 4 ml methanol was added and the mixture evaporated. 35 ml saturated NaHCO3 solution was added. The precipitate was filtered, washed with water and dried, recrystallized from methanol giving 0.37 g of title compound, melting at 195-8° C.
| WO2005012266A1 * | May 21, 2004 | Feb 10, 2005 | Richter Gedeon Vegyeszet | (thio) carbamoyl-cyclohexane derivatives as d3/d2 receptor antagonists |
| WO2008142461A1 * | May 15, 2008 | Nov 27, 2008 | Richter Gedeon Nyrt | Metabolites of (thio)carbamoyl-cyclohexane derivatives |
| WO2010070370A1 * | Dec 18, 2009 | Jun 24, 2010 | Richter Gedeon Nyrt. | Process for the preparation of piperazine compounds and hydrochloride salts thereof |
| WO2010070371A1 * | Dec 18, 2009 | Jun 24, 2010 | Richter Gedeon Nyrt. | Process for the preparation of piperazine derivatives |
| HU0302451A2 | Title not available |
References
- Kiss B; Horváth A; Némethy Z; Schmidt E; Laszlovszky I; Bugovics G; Fazekas K; Hornok K; Orosz S; Gyertyán I; Agai-Csongor E; Domány G; Tihanyi K; Adham N; Szombathelyi Z (2010). “Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile”. The Journal of Pharmacology and Experimental Therapeutics 333 (1): 328–340. doi:10.1124/jpet.109.160432. PMID 20093397.
- Gründer G (2010). “Cariprazine, an orally active D2/D3 receptor antagonist, for the potential treatment of schizophrenia, bipolar mania and depression”. Current Opinion in Investigational Drugs 11 (7): 823–832. PMID 20571978.
- Clinical trial : Safety and Efficacy of Caripazine As Adjunctive Therapy In Major Depressive Disorder
- Citrome, L (February 2013). “Cariprazine: chemistry, pharmacodynamics, pharmacokinetics, and metabolism, clinical efficacy, safety, and tolerability”. Expert Opinion on Drug Metabolism and Toxicology 9 (2): 193–206. doi:10.1517/17425255.2013.759211. PMID 23320989.
- Citrome L (February 2013). “Cariprazine in schizophrenia: clinical efficacy, tolerability, and place in therapy”. Adv Ther 30 (2): 114–26. doi:10.1007/s12325-013-0006-7. PMID 23361833.
- Veselinović T, Paulzen M, Gründer G (November 2013). “Cariprazine, a new, orally active dopamine D2/3 receptor partial agonist for the treatment of schizophrenia, bipolar mania and depression”. Expert Rev Neurother 13 (11): 1141–59. doi:10.1586/14737175.2013.853448. PMID 24175719.
- Newman-Tancredi, A.; Kleven, MS. (Aug 2011). “Comparative pharmacology of antipsychotics possessing combined dopamine D2 and serotonin 5-HT1A receptor properties”.Psychopharmacology (Berlin) 216 (4): 451–73. doi:10.1007/s00213-011-2247-y. PMID 21394633.
- Gyertyán, I.; Kiss, B.; Sághy, K.; Laszy, J.; Szabó, G.; Szabados, T.; Gémesi, LI.; Pásztor, G. et al. (Nov 2011). “Cariprazine (RGH-188), a potent D3/D2 dopamine receptor partial agonist, binds to dopamine D3 receptors in vivo and shows antipsychotic-like and procognitive effects in rodents”. Neurochemistry International 59 (6): 925–35.doi:10.1016/j.neuint.2011.07.002. PMID 21767587.
- Seeman, P.; Kapur, S. (Jul 2000). “Schizophrenia: more dopamine, more D2 receptors”. Proceedings of the National Academy of the Sciences of the United States of America 97 (14): 7673–5. PMC 33999. PMID 10884398.
- Seneca, N.; Finnema, SJ.; Laszlovszky, I.; Kiss, B.; Horváth, A.; Pásztor, G.; Kapás, M.; Gyertyán, I. et al. (Dec 2011). “Occupancy of dopamine D₂ and D₃ and serotonin 5-HT₁A receptors by the novel antipsychotic drug candidate, cariprazine (RGH-188), in monkey brain measured using positron emission tomography”. Psychopharmacology (Berlin) 218 (3): 579–87.doi:10.1007/s00213-011-2343-z. PMC 3210913. PMID 21625907.
- Citrome, L (February 2013). “Cariprazine in Schizophrenia: Clinical Efficacy, Tolerability, and Place in Therapy”. Advances in Therapy 30 (2): 114–126. doi:10.1007/s12325-013-0006-7.PMID 23361833.
- Domany, G.
Discovery of novel dopamine D3/D2 ligands for the treatment of schizophrenia
234th ACS Natl Meet (August 19-23, Boston) 2007, Abst MEDI 383
| WO1996007331A1 * | Sep 8, 1995 | Mar 14, 1996 | Helena Halttunen | Composition comprising co-crystals, method for its manufacture, and its use |
| US20090023750 * | May 9, 2008 | Jan 22, 2009 | Richter Gedeon Nyrt. | Novel salts of piperazine compounds as d3/d2 antagonists |
| US20090030007 * | May 9, 2008 | Jan 29, 2009 | Forest Laboratories Holdings Limited | crystalline form of trans-1 {4-[2-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-ethyl]-cyclohexyl}-3,3-dimethyl-urea hydrochloride (Form III) Cariprazine {RGH-188); Dysfunction of the dopaminergic neurotransmitter system is involved in the pathology of several neuropsychiatric and neurodegenerative disorders |
| US20090054455 * | Feb 2, 2007 | Feb 26, 2009 | Dr. Reddy’s Laboratories Ltd. | Aripiprazole co-crystals |
| US20100137335 * | May 15, 2008 | Jun 3, 2010 | Eva Againe Csongor | Metabolites of (thio) carbamoyl-cyclohexane derivatives |
Richter Gedeon Gyógyszergyár


Filed under: Phase3 drugs, Uncategorized Tagged: CARIPRAZINE, Gedeon Richter, Major Depressive Disorder, PHASE 3, schizophrenia










