
Fanetizole
Fanetizole shows immunoregulating activity.
RN: 79069-95-7
Fanetizole mesylate [USAN]
CP-48,810-27
Fanetizole mesylate
UNII-D3OG7B0G4M
Synthesis
Thioureas serve as a convenient starting material for 2-aminothiazoles.
Reaction of β-phenethylamine with ammonium isothiocyanate gives the corresponding thiourea. Treatment of that product with phenacyl bromide thus affords the thiazole product.[1]
- Lombardino, J. G.; 1981, U.S. Patent 4,307,106
| Systematic (IUPAC) name | |
|---|---|
| 4-Phenyl-N-(2-phenylethyl)-1,3-thiazol-2-amine | |
| Clinical data | |
| Legal status |
?
|
| Pharmacokinetic data | |
| Protein binding | % |
| Identifiers | |
| CAS number | 79069-94-6 |
| ATC code | ? |
| PubChem | CID 54339 |
| ChemSpider | 49083 |
| UNII | BH48F620JA  |
| Chemical data | |
| Formula | C17H16N2S |
| Mol. mass | 280.39 g/mol |
………………………………………….
Journal of the Chinese Chemical Society, 2009, 56, 455-458
http://proj3.sinica.edu.tw/~chem/servxx6/files/paper_10990_1246593848.pdf
Fanetizole (3j)
mp 114-115 C (Lit.,30 116-117 C). IR (KBr) :3192, 2957, 1562, 1481, 1445, 1332, 698 cm-1;
1H NMR(CDCl3) : 2.81 (t, J = 7.4 Hz, 2H), 3.42 (dd, J = 6.8, 10.8
Hz, 2H), 6.32 (s, 1H), 6.64 (s, 1H), 7.08 (d, J = 6.8 Hz, 2H),
7.15-7.28 (m, 4H), 7.34-7.37 (m, 2H), 7.77-7.80 (m, 2H).
30=. Potewar, T. M.; Ingale, S. A.; Srinivasan, K. V. Tetrahedron
2008, 64, 5019-5022.
…………………………………………
A remarkably high-speed solution-phase combinatorial synthesis of 2-substituted-amino-4-aryl thiazoles in polar solvents in the absence of a catalyst under ambient conditions and study of their antimicrobial activities
ISRN Organic Chemistry (2011), 434613, 6 pp. Publisher: (Hindawi Publishing Corp., )
http://www.hindawi.com/journals/isrn/2011/434613/
……………………………………………
Fanetizole
Ley et al had previously developed a tube-in-tube reactor based on a semipermeable polymer membrane to enable the transfer of gases into liquid flow streams. and here, we demonstrate the scalability and throughput of this reactor when used with ammonia gas. This was made possible by a the inclusion of a titration method to assess parameters including the liquid and gas configuration, reactor temperatures, flow rates, and solvent polarity. These data were then employed in a scaling-up process affording alkyl thioureas which were ultimately used in a telescoped procedure for the preparation of anti-inflammatory agent fanetizole on a multigram scale.
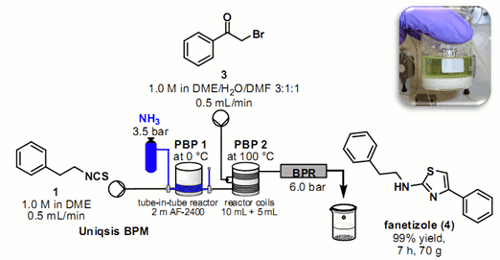
Researchers at Cambridge have shown how it is possible to calibrate a ‘tube-in-tube’ reactor containing ammonia gas using a simple in-line colourimetric titration technique.
This information was then used to deliver an ammonia solution of stoichiometrically to effect the telescoped 2 stage synthesis of the anti-inflammatory agent Fanetizole.
The automated continuous flow synthesiser was able to produce drug substance at a rate of approximately 10 g per hour, isolating the product by direct precipitation from the outflow reaction stream.
Fanetizole: Scaling-up of continuous flow processes with gases using a tube-in-tube reactor: in-line titrations and fanetizole synthesis with ammonia J. Pastre, D.L. Browne, M. O’Brien and S.V. Ley, Org. Proc. Res. Dev. 2013, 17, 1183-1191.
http://pubs.acs.org/doi/full/10.1021/op400152r
………………………..
A Hantzsch synthesis of 2-aminothiazoles performed in a heated microreactor system
DOI: 10.1039/B109360F…….http://pubs.rsc.org/en/content/articlelanding/2002/lc/b109360f/unauth#!divAbstract
………………………………
Bioorganic and Medicinal Chemistry Letters, 1996 , vol. 6, 12 pg. 1409 – 1414
http://www.sciencedirect.com/science/article/pii/0960894X96002417

………………………………………
ref
Heterocycles, 2010 , vol. 81, 12 pg. 2849 – 2854
Journal of the Chinese Chemical Society, 2009 , vol. 56, 3 pg. 455 – 458
Bioorganic and Medicinal Chemistry Letters, 1996 , vol. 6, 12 pg. 1409 – 1414
Pfizer Patent: DD144055DE2922523 , 1979 ;Chem.Abstr., vol. 92, 111001
Organic Process Research and Development, 2013 , vol. 17, 9 pg. 1183 – 1191
Tetrahedron, 2007 , vol. 63, 45 pg. 11066 – 11069
Tetrahedron, 2008 , vol. 64, 22 pg. 5019 – 5022
Filed under: Uncategorized Tagged: fanetizole


